June 1, 2014 (Vol. 34, No. 11)
Transfection, a key genetic technology in the toolbox of many researchers, spans numerous applications—production of recombinant proteins and recombinant cell lines, drug discovery, delivery of therapeutics, and gene therapy. Protocols and techniques for coaxing nucleic acids into cells vary widely. They include biological, physical, and chemical methods.
The global market for transfection technologies, valued at $385 million in 2012, is expected to reach $601 billion by 2017. These figures reflect a compound annual growth rate of 9.32%.
Although widespread and successful, key challenges remain in the field, such as how to optimize performance, reduce toxicity, and penetrate difficult-to-transfect cells. Researchers are tackling these issues by developing new reagents, refining transfection systems, and modifying cellular models.
Novel Bioproduction Reagent
Chinese hamster ovary (CHO) cells are often used for production of high-quality recombinant proteins and antibodies. Although use of a well-characterized, clonal stable cell line is the de facto standard for bulk production of therapeutics, often transient transfection is preferred for research, discovery, and early-stage preclinical studies.
“A major bottleneck in transient transfection of CHO cells is low transfection efficiency and batch-to-batch variation,” notes Géraldine Guérin-Peyrou, a bioproduction product specialist at Polyplus-transfection. “Our company focuses on nontoxic, animal-free transient transfection reagents adapted to a wide range of applications from academic research to pharmaceutical bioproduction and therapeutics. We synthesize in-house and provide formulated, tested, and qualified reagents for high-level production of recombinant proteins.”
Different steps play critical roles in transfection. “First, the cationic transfection reagent associates with DNA and forms positively charged complexes that interact with anionic proteoglycans on the cell membrane,” explains Guérin-Peyrou. “The transfection complex is then internalized and shuttled to endosomes where DNA has to be protected from degradation.”
“The unique property of our products is their ability to buffer the pH of the endosomal compartment,” adds Guérin-Peyrou. “This mechanism ultimately leads to rupture of the endosome, release of the complexes into the cytoplasm, and ultimately nuclear transport that allows transcription.”
The company also has its eye on two new trends in the industry: increasing productivity and reducing the amount of DNA needed for efficient transfection. According to Guérin-Peyrou, the company has just launched FectoPRO™, a new product for bioproduction of protein and antibodies using transient gene expression (TGE) in mammalian cells. “FectoPR allows for very high protein production yields while using half the amount of DNA,” she asserts. “Our line of products for bioproduction also includes PEIpro™, a cost-effective linear PEI-based transfection reagent specially designed for large-scale bioproduction of proteins and therapeutic viruses.”
The improvement in protein production yields and the higher quality of transfection reagents is aimed at allowing transient transfection for future therapeutic protein and virus production, providing more flexibility and higher safety. In fact, as Guérin-Peyrou points out, the company anticipates that “influenza viruses could be generated by TGE in the upcoming years.”
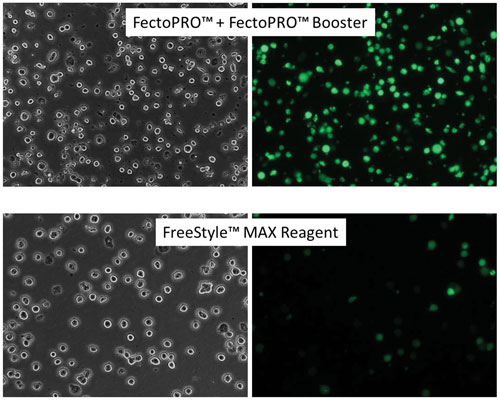
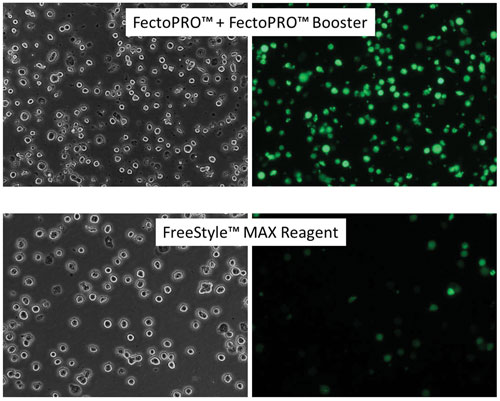
Polyplus-transfection develops solutions for the delivery of nucleic acids. For example, the company’s FectoPRO™ can give high transfection efficiency in suspension CHO cells. FreeStyle™ CHO-S cells were seeded at 1 × 106 cells/mL in 30 mL of FreeStyle CHO Expression Medium and transfected with FectoPRO Booster or FreeStyle MAX Reagent using 0.4 µg/mL and 1.25 µg/mL of a GFP-expressing plasmid, respectively. GFP expression was assayed 24 hours after transfection using fluorescence microscopy. (FreeStyle is a trademark of Life Technologies.)
Flow Electroporation
Maintaining cell background from screening through process development and into production can greatly accelerate protein development by ensuring that early-stage analytical and activity data are indicative of the final manufactured product. Compared to generating stable cell lines, transient transfection offers multiple efficiencies for characterizing proteins. The ideal transient transfection system should provide the scalability to easily and rapidly produce from small to large quantities of protein.
“Our transfection platform, based on proprietary flow electroporation technology, enables scalable transient transfections from half a million to more than 200 billion cells,” says James Brady, Ph.D., director of technical applications at MaxCyte.
Electroporation applies an electric field to suspended cells and briefly causes the cellular membrane to become reversibly permeable to DNA, RNA, proteins, etc. In contrast to conventional electroporation, flow electroporation streams cells through an electroporation chamber.
“We typically achieve greater than 95% transfection efficiency and viability at both small and large scales, asserts Dr. Brady. “Further, working with CHO cells, which are challenging to transfect with conventional methods, the system can produce antibody titers greater than 1 gram per liter with post-transfection optimization, yielding multiple grams of antibody from a single transfection.”
The MaxCyte STX® Scalable Transfection System and MaxCyte VLX® Large Scale Transfection System are preloaded with protocols optimized for more than 60 cell types. “The system is compatible with most cell lines commonly used for bioproduction,” continues Dr. Brady. “It also enables cell-based assay development with physiologically relevant cells. The MaxCyte platform doesn’t require any specialized constructs, engineered cells, or media.”
The company is expanding its technology applications for direct transfection of insect cells to avoid the cumbersome route of baculoviral expression. Additionally, it is using flow electroporation for vaccine development and manufacturing multiple-gram quantities of recombinant protein antigens, virus-like particles, viral vectors, and viruses for tens of thousands of doses in a single run.
Membrane Expression
Membrane proteins, such as receptors, channels, and transporters, play critical roles in a wide variety of biological processes. Because of their highly hydrophobic and intricate structures, one bottleneck in studying membrane proteins is the difficulty expressing them in sufficient quantities as properly folded and stable proteins. Often multiple rounds of protein engineering are required to optimize the process, which could take a year or longer to perfect.
“Many researchers use the baculovirus-based expression system because, unlike bacterial systems, it adds appropriate post-translational modifications required for proper folding and function,” says Hao Chen, Ph.D., senior scientist, protein technologies, Amgen.
Dr. Chen and his team developed a method for expressing proteins in baculovirus that modifies the traditional method to dramatically reduce time and resources. “Baculovirus manipulations generally require creating, titering, and amplifying viral stocks that are used to express proteins in insect cells,” notes Dr Chen. “This is a laborious process that takes about 3–4 weeks. We developed a method to screen membrane protein expression that typically takes only 2–3 days.”
The team made expression plasmids that fused green fluorescent protein (GFP) to the target membrane proteins and transfected the construct directly into insect cells without going through typical viral approaches.
“Transient expression of recombinant proteins in insect cells has not been widely adopted due to its low protein yield and difficulty scaling up. However, by fusing GFP to the membrane protein, we can directly monitor the resulting fusion proteins in whole cells for their subcellular localizations using fluorescence microscopy,” continued Dr. Chen. “Additionally, we use a method called fluorescence-detection size exclusion chromatography, or FSEC, that requires only nanogram levels of unpurified protein to characterize expression level and approximate molecular size and stability of the over-expressed integral membrane proteins.”
Dr. Chen utilized the ion-channel protein ASIC3 and transporter SLC7A5 to demonstrate the technique. “If a researcher has an HPLC capability, this technique is super easy and ultrasensitive,” asserts Dr. Chen.
The challenge of the technique is its limited throughput. Still, according to Dr. Chen, many people have shown great interest in the method because it provides a way to “tackle difficult or poorly characterized proteins, the number of which continues to grow.”
Polymeric Formulation
There are many barriers to transfection at a cellular level including the plasma membrane, the endosome membrane, and nuclear trafficking and entry (for plasmid DNA). According to Laura Juckem, Ph.D., R&D group leader, Mirus Bio, “Cell types of different lineages have different tolerances for the internalization of foreign nucleic acid as evidenced by their varying transfection efficiencies.
“Furthermore, a high-performance delivery solution for both plasmid DNA and smaller nucleic acids such as siRNA/miRNA has not been available to researchers; this is primarily due to the molecular weight and charge differences between plasmid DNA and siRNA that create hurdles for reagent design and co-delivery.”
Through fine-tuning of chemical structures and high-throughput screening, Mirus Bio scientists have identified a novel, non-liposomal polymeric formulation, the TransIT-X2™ Dynamic Delivery System. “Using this system, we are able to dynamically coat and condense plasmid DNA and/or siRNA and miRNA to overcome nucleic acid uptake and endosomal release barriers,” notes Dr. Juckem.
“High protein expression or gene silencing is observed in an expansive number of cell types including primary cells such as Human Umbilical Vein Endothelial Cells (HUVEC) and Human Mammary Epithelial Cells (HMEC).”
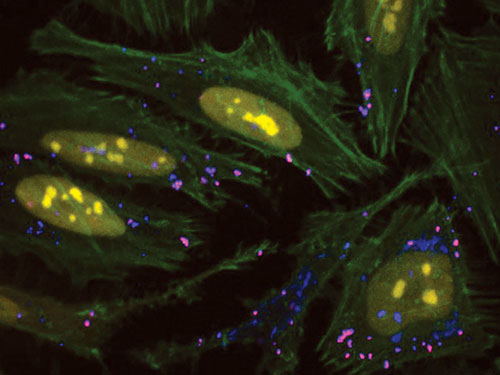
Functional co-delivery of plasmid DNA and siRNA was accomplished using Mirus Bio’s TransIT-X2™ Dynamic Delivery System, which transfected plasmid Cy®5-labeled DNA encoding nuclear YFP and Cy®3-labeled siRNA into HeLa cells. Transfection was performed in six-well plates with poly-L-Lysine (PLL)-coated coverslips using 4 µL of TransIT-X2 to deliver 2 µg of DNA and 25 nM siRNA (2:1 reagent:DNA ratio). Actin cytoskeleton was stained using Alexa Fluor® 350 phalloidin. Images (63×) were captured at 24 hours post-transfection using a Nikon A1R confocal microscope. Yellow: nuclear YFP; blue: Cy5-labeled DNA;, red: Cy3-labeled siRNA); green: actin cytoskeleton.
Adapted Cell Lines
According to Henry J. George, R&D manager, SAFC, “The biggest improvement needed for transfection technology is efficiency. Many cell lines are either hard to transfect and/or have low efficiency (less than 10–20%), so getting higher transfection efficiency is the key to successful downstream experimentation.”
However, there are challenges to creating efficiency. These, say George, are “due to the various cells lines, growth media, and conditions being used, as well as the payload (such as DNA, RNA, and protein) being transported into the cells. Additionally, cell culture growth conditions and harvest time for optimal production are not yet standardized protocols. No ‘one size fits all’ approach is available.”
SAFC is tackling this issue by development of larger-scale transient transfection of suspension cell lines cultured under chemically defined media conditions. “Our Sigma-Aldrich CHOGS and CHODHFR, which are specially engineered CHO-K1 cells,” asserts George, “are suspension-adapted cell lines that show higher-percentage transfection efficiencies with electroporation.”
Addressing Toxicity
Although many current methods work well for the efficient transfection of standard cell models, the issue of toxicity associated with some reagents remains, suggests Kevin Kopish, strategic marketing manager, cellular analysis, Promega.
“There is also a trend toward more physiologically relevant model systems, such as primary and stem-cell derived cells, that are increasingly more difficult to transfect with existing methods and are much more sensitive to the toxicity of transfection reagents, Kopish notes. “Earlier this year, Promega released the ViaFect™ Transfection Reagent to address this need. With this offering and the FuGENE® 6 and FuGENE HD products, there are reagents that can transfect a great diversity of cell types with little impact on cell viability.”
Kopish also cites the effect of expression levels after transfection: “Many transfection systems cause gross overexpression of exogenous proteins that can overwhelm the cells and mask true biological responses. We have therefore developed an ultra-sensitive reporter called NanoLuc luciferase that excels in studying tagged proteins even when expressed at or below physiological levels. This sensitivity also allows better performance in systems with lower transfection efficiency since the reporter can still provide large detectable signal.”
Off-Target Effects
“Transfection-mediated cytotoxicity and off-target effects are often overlooked, but they are serious considerations as they create an unknown bias in experimental interpretation,” advises Lauren Buck, Ph.D., international marketing manager, Roche. Dr. Buck indicates that high transfection efficiency with minimal cytotoxicity is easily obtainable with Roche’s X-tremeGENE Transfection Reagents, even in many primary and tumor cell lines.
“X-tremeGENE Reagents are nonliposomal formulations and had negligible cytotoxic effects on cells when tested in real-time cell monitoring studies,” asserts Dr. Buck. “Also, in a recent study, minimal off-target effects (≤1%) were detected in 221 apoptosis-related genes using LightCycler® qRT-PCR. Researchers can have confidence in the physiologically relevant data they generate with overexpression studies using X-tremeGENE 9 and HP Transfection Reagents.”
Dr. Buck adds that as demands on researchers increase, it becomes crucial to have the correct application and cell line-specific protocol information available: “Having the correct transfection technology is meaningless unless it can be effectively implemented in the lab.”
Roche has generated an extensive database of researcher-submitted protocols for X-tremeGENE reagents. Filtering by cell line, application, or by transfected material allows one to home in on the information needed for projects of interest.
Biologically Relevant Models
Many basic research questions are still first addressed by transfecting cell lines which are easy to access and easy to culture, but also artificially immortalized or cancer-derived. “To confirm that insights gained from those ‘artificial’ cell line systems reflect the in vivo situation, there is a need to switch to more biologically relevant models,” suggests Andrea Toell, Ph.D., product manager for transfection, Lonza.
“For therapeutic approaches, use of primary animal cells could reduce the number of tests in animals, and use of primary human cells allows the extrapolation of findings from such animal models,” continues Dr. Toell. “Another trend that is changing transfection needs toward an increased use of primary cells or stem cells is cell therapy.”
According to Dr. Toell, a growing number of cell-therapy approaches require the ex vivo transfection of human embryonic or adult stem cells, or primary cells (such as human T cells or dendritic cells) in large numbers: “Moreover, using nonviral methods for reprogramming somatic cells into induced pluripotent stem cells (iPSC) and for engineering those iPSCs are key prerequisites for transferring the use of iPSC from basic research to cell-therapy approaches.”
Lonza’s electroporation-based Nucleofector™ Technology is a nonviral technology that can achieve much higher transfection efficiencies and viabilities for hard-to-transfect cells, such as primary cells or stem cells. “Transfection reagents require cell proliferation for transferring the DNA into the nucleus for expression,” notes Dr. Toell. “With the Nucleofector Technology, the DNA is directly transferred to the nucleus and enhances transfection of nondividing cells, such as unstimulated T cells or neurons.”
Counting Cells Accurately
Optimizing transfection efficiency requires an accurate and consistent number of input cells, according to Steve Kulish, managing director, cell biology business unit, Bio-Rad Laboratories. “Researchers traditionally count cells under the microscope using a hemocytometer,” says Kulish, “which is very subjective, tedious, unreliable, and extremely labor intensive.”
According to Kulish, Bio-Rad’s TC20 automated cell counter offers a simple, rapid, and reproducible alternative to the hemocytometer: “The TC20 completes the counting of mammalian cells—suspended or re-suspended adherent—in one simple step, initiating a count immediately upon slide insertion.”
Cell counters that use a single plane of focus to assess cell viability may lack accuracy, but the TC20 scores each sample across multiple planes. “The auto-focus technology and image analysis algorithm provide accurate and reproducible counts in less than 30 seconds,” notes Kulish. “Cell viability can also be assessed with the use of trypan blue, a dye that is excluded from live cells and incorporated into dead cells.”