November 15, 2010 (Vol. 30, No. 20)
Homogeneous Nonradioactive Assays to Monitor Methylation Process
The expression of the thousands of genes present in the human cell is regulated by dynamic changes in chromatin compaction throughout the different regions of the genome. The activity of chromatin-remodeling machineries, as well as changes in DNA-methylation patterns and histone post-translational modifications by specialized enzymes, are all biochemical strategies that cells employ to modulate gene expression.
For instance, DNA methylation on CpG islands as well as methylation of histone H3 on specific lysine residues are events associated with chromatin condensation leading to transcriptional repression. Conversely, lysine acetylation of histones is generally related to open chromatin structures with higher rates of transcription.
In this regard, there is a growing body of evidence that associates the activity of both histone methyltransferases (HMTs) and acetyltransferases (HATs) with different human pathologies, from malignancies to neuropathies. Thus, the development of molecular probes for these enzyme families could enable biologists to study in more depth the complex role of histone modifications in gene regulation, and provide a starting point to new drug discovery.
A variety of radioactive-based assays have been reported in the literature for identifying compounds that modulate the activity of histone-modifying enzymes. Scintillation proximity assays (SPA) and FlashPlate® have been successfully developed to monitor the activity of HMTs by measuring the transfer of the methyl group from the radiolabeled co-factor, S-adenosyl-L-methionine (SAM), to the ε-amino residue of lysines on histone-derived peptidic substrates. However, as HMTs can perform either mono-, di-, or tri-methylation of lysines, measuring the incorporation of radioactive methyl groups does not provide information on the nature of the final reaction products.
Mass spectroscopy (MS) is a non-radioactive alternative that has also been employed for studying the activity of HMTs and HATs. This technology allows the simultaneous characterization of the different methyl-lysine forms produced by certain HMTs, i.e., mono-, di-, or tri-methylated lysines. However, while recent technical advancements continue to improve MS throughput, plate-based detection assays remain the standard for speed and sensitivity in HTS applications.
Simplifying Hit Discovery
PerkinElmer’s Lance® Ultra and AlphaLISA® homogeneous assay platforms are two non-radioactive antibody-based technologies that have been demonstrated to be novel tools for the study and compound screening of a variety of enzymatic targets.
Specific reagents for epigenetic research have now been developed for these platforms, to detect the in vitro enzymatic modification of histone H3-derived substrates (lysine acetylation or methylation). These microtiter plate assays consist of two main steps: the enzymatic reaction is performed first, followed by the detection of reaction products.
During the enzymatic reaction step, appropriate concentrations of enzyme and biotinylated peptide substrate are incubated together in the presence or absence of test compounds. Following this, antimark europium-labeled antibody and ULight-labeled streptavidin (for Lance Ultra assay), or antimark AlphaLISA Acceptor beads and streptavidin-Donor beads (for AlphaLISA assays), are added to the reaction mix for product detection.
The Lance Ultra and AlphaLISA reagents have been subjected to extensive optimization that includes a thorough analysis of the specificity of the antibody used to capture the modified residue. It is of note that for the AlphaLISA platform, covalent conjugation of antibodies to the Acceptor beads generally increased specificity and stability of the signal, when compared with an indirect capture of the antibody by Acceptor beads conjugated to an antispecies antibody. Moreover, the use of Acceptor bead-conjugated antibodies decreases background levels significantly, allowing for improved assay windows.
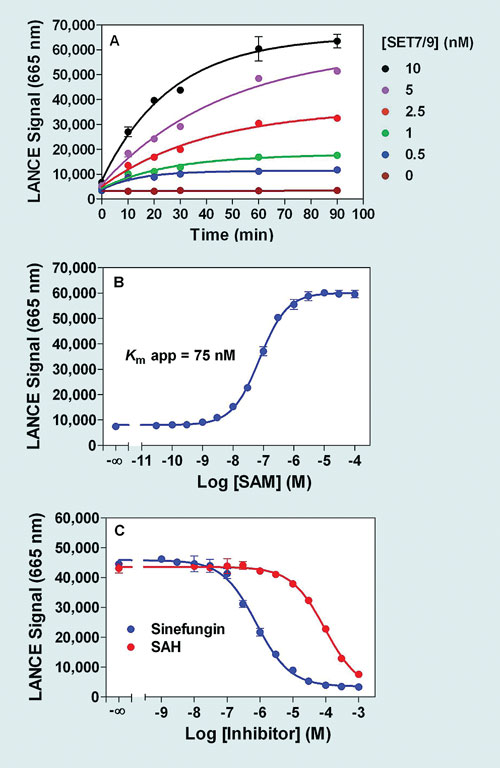
Figure 1. Lance Ultra SET7/9 methyltransferase assay optimization: All enzymatic reactions were performed in 10 µL (final assay volume: 20 µL). Detection has been carried out with an Eu-labeled antibody specific against the mono- and di-methylated forms of the Histone H3 peptide substrate (H3K4me1-2). (A) Enzymatic time course, at 200 nM biotin-H3 peptide substrate, and 100 µM SAM (saturating concentration). (B) SAM dose-response curve (5 nM SET7/9, 200 nM biotin-H3 peptide substrate). (C) Inhibitor dose-response curves (5 nM SET7/9, 200 nM biotin-H3 peptide substrate, 300 nM SAM).
Determination of optimal enzymatic assay conditions has to be performed for each enzyme studied. Figures 1 and 2 provide examples of results obtained following the successful optimization of a SET7/9 assay (H3 Lys4 methylation) using either Lance Ultra or AlphaLISA detection platforms. With both assays, signal increase was proportional to enzyme reaction time and enzyme concentration (Figure 1A and 2A). Apparent Km values of 75 and 79 nM were obtained for the SAM co-substrate in Lance Ultra and AlphaLISA assays, respectively (Figure 1B and 2B).
In addition, rank order of potency for two known methyltransferase inhibitors (SAH, sinefungin) was as expected and further validated the assay suitability for studying small molecule inhibitors (Figure 1C and 2C). Low intra-assay variability was confirmed by Z´-factor values over 0.8, generated with both technologies in a study using maximal inhibition with sinefungin (data not shown).
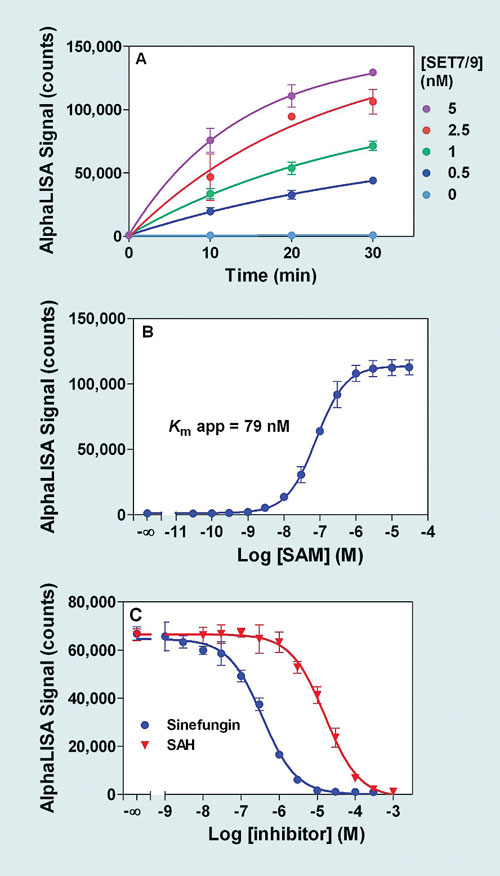
Figure 2. AlphaLISA SET7/9 methyltransferase assay optimization: All enzymatic reactions were performed in 10 µL (final assay volume: 25 µL). Detection has been carried out with Acceptor beads conjugated to an antibody specific against the mono- and di-methylated forms of the Histone H3 peptide substrate (H3K4me1-2). (A) Enzymatic time course, at 50 nM biotin-H3 peptide substrate, and 100 µM SAM (saturating concentration). (B) SAM dose-response curve (1 nM SET7/9, 50 nM biotin-H3 peptide substrate). (C) Inhibitor dose-response curves (1 nM SET7/9, 50 nM biotin-H3 peptide substrate, 100 nM SAM).
Conclusions
As exemplified in this SET7/9 study, peptide substrate and enzyme concentrations in the nanomolar range were sufficient to generate robust and reproducible assay signals using either Lance Ultra or AlphaLISA reagents. Figure 3 shows a scheme of how the signal is produced on both platforms. This represents a significant advantage over other technologies using colorimetric or fluorescence-based detection methods that generally require micromolar substrate concentrations.
More significantly, our Lance Ultra and AlphaLISA reagents allowed the use of mid-nanomolar concentrations of SAM, facilitating the screening of SAM competitive inhibitors.
The Lance Ultra europium-antibodies and AlphaLISA Acceptor beads developed for epigenetic research are available as stand-alone reagents, allowing the user maximum flexibility for assay development with preferred substrate and enzyme source. Due to the simple all-in-one-well format, the small number of steps involved (usually 3 or 4), and the absence of wash steps, Lance Ultra and AlphaLISA assays for epigenetic research are amenable to automation. For all these reasons, they constitute a good choice not only for HTS campaigns (hit finding) but also for studies involving secondary screenings and orthogonal testing (hit-to-lead studies).
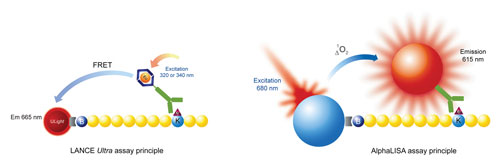
Figure 3. Lance Ultra and AlphaLISA platforms in the detection of histone modifications: Detection of epigenetic modifications on a biotinylated peptide substrate can be accomplished using either the Lance Ultra (TR-FRET) or AlphaLISA (proximity-based chemiluminescent) platforms. In Lance Ultra, excitation at 320–340 nm of a Europium chelate conjugated antimark antibody enables the resonance energy transfer to the acceptor ULight dye conjugated to streptavidin. This results in the emission of photons at 665 nm. In AlphaLISA, excitation of the streptavidin Donor beads at 680 nm triggers the release of short-lived singlet oxygen molecules that activate the Acceptor beads in close proximity to emit light at 615 nm. In both cases, the signal output is proportional to the amount of modified biotin-peptide product.
Roberto Rodriguez-Suarez, Ph.D. ([email protected]), is senior scientist, and Nathalie Rouleau ([email protected]) is R&D section leader, biochemical assay at PerkinElmer.



