May 1, 2011 (Vol. 31, No. 9)
Simple Technique Can Be Leveraged Throughout Drug Development Process
Dried blood spot (DBS) technology, a very simple technique for collecting, shipping, and storing blood samples, is widely used in applications such as screening for metabolic and sickle cell disorders, and HIV and malarial infections. The method involves spotting 15 μL of blood from a finger or heel prick onto absorbent filter paper that is then shipped and stored at room temperature (Figure 1). For analysis, the blood spot is punched out of the filter paper and the blood is extracted using a solvent (typically methanol) containing an internal standard.
Although DBS has been in existence for almost 50 years, the technology could only be used to screen for the presence or absence of a particular marker—a simple yes or no answer. It could not be used to determine to what extent a marker is present because the analytical technologies were not sensitive enough to obtain reliable, quantitative data from such tiny blood samples.
It is for this reason that DBS has not been widely utilized within drug discovery and development process. However, recent advancements that have been made in the sensitivity of analytical technologies—mainly mass spectrometry and ultra-high-performance liquid chromatography—have overcome this limitation. As a result, there is heightened interest within the pharmaceutical industry to replace liquid blood sampling with DBS.
DBS sampling can offer enormous advantages over liquid blood or plasma in both preclinical and clinical studies. These advantages include, but are not limited to, a significant reduction in the volume of blood collected, a simplified process that does not require the need to centrifuge, sub-aliquot, freeze, and defrost samples (all of which can introduce errors in the analysis), improved safety with handling, shipping, and storage at room temperature, improved data quality, improved compound stability for drugs and their metabolites, and considerable cost savings.

Figure 1. The DBS technique involves spotting blood from a finger or heel prick onto absorbent filter paper that is then shipped and stored at room temperature.
Preclinical Advantages
The use of DBS in preclinical studies results in a fivefold reduction in the volume of blood collected, which has a significant impact on animal studies and data quality (Figure 2). The number of rodents needed for each study can be reduced by up to 75%, and because fewer animals are needed, the quantity of compound needed for testing is also greatly reduced. The quantity of compound required for animal studies is very important in the early stages of drug development when the synthesis of the compound has not yet been optimized and is costly, time-consuming, and difficult to achieve.
DBS sampling also contributes to the generation of higher quality data in pre-clinical studies because more time points can be added without the need for additional rodents and the technology allows for serial pharmacokinetic (PK) profiling. Serial PK profiling eliminates the variability between animals observed when using composite profiling and greatly improves the quality of the data. In addition, DBS allows for pre-clinical juvenile toxicology studies to be conducted in small animals where the availability of blood has always been a problem. These studies are always necessary and required by regulatory agencies in support of clinical pediatric studies.
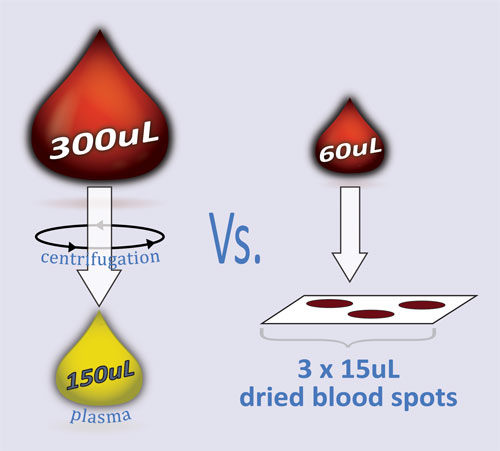
Figure 2. The use of DBS sampling results in a fivefold reduction in blood volume and improved data quality.
Clinical Advantages
As drug development programs progress into the clinic, the continued use of DBS provides additional benefits and cost savings. The simplified, less invasive blood sampling (finger/heel prick) is much more patient friendly than blood draws, especially for pediatric studies and in critically ill patients. The shipping, handling, and storage costs are also greatly reduced because DBS is safe and can be stored at room temperature.
Unlike liquid blood or plasma, DBS does not need to be handled as a biohazard since pathogens like HIV and hepatitis B are inactivated. Biohazardous samples are not only expensive to ship, but special training and licensing are also required. In addition, certain countries will not let blood samples be sent out of the country because they are biohazardous. The use of DBS can overcome these barriers.
Sample handling is much easier using DBS because the sample does not need to be centrifuged for plasma harvesting and then transferred to secondary tubes for freezing. Finally, refrigeration is not needed during transport or for storage as the DBS can be stored at room temperature. Normally, specialized couriers are needed to ship samples in dry ice, and for large Phase II/III clinical trials that require thousands of samples to be shipped from different sites, this process can be very expensive using liquid blood. The reduced amount of specialized equipment at clinical sites (refrigerated centrifuge, monitored freezers, etc.) also makes DBS technology extremely valuable when conducting clinical studies in emerging countries.
Increasingly Sensitive Technologies
At Aptuit, we have developed and fully validated more than 50 preclinical and clinical assays using DBS for different classes of compounds, some of which have been used in support of regulatory studies. In addition, we have developed an assay for a commercial drug that has demonstrated extremely high sensitivity—five picograms per mL. To achieve this sensitivity, we had to use hydrophilic interaction liquid chromatography (HILIC), which is a version of normal-phase liquid chromatography that offers a significant increase in sensitivity in mass spectrometry analysis over conventional techniques. Although HILIC technology is not new, the use of HILIC in DBS analysis represents an innovative approach.
We have demonstrated that the use of HILIC in DBS analysis can overcome the biggest limitation of DBS—difficulty reaching the lower limit of quantification levels due to limited sample volume. The development and validation of this clinical method provides evidence that the poor sensitivity of analytical assays, which made DBS inapplicable in extremely low dose clinical studies, is now less than an issue.
Overcoming Limitations
The limitations of DBS relate to the small size of the samples collected. Bioanalytical method development and validation is more laborious because various card types and additional parameters need to be assessed. Target sensitivity may be an issue for compounds that are administered at a very low therapeutic dose or those that are inhaled. In addition, it may be very difficult—and risky—for companies to switch from blood draws to DBS for clinical studies if blood draws were used in the preclinical stage of development because the data obtained is not directly comparable. In such cases bridging studies would be necessary, but regulatory agencies have not provided guidance on such an approach.
We have developed a new technique called dried plasma spotting (DPS) that collects plasma on filter paper instead of whole blood. DPS, which can be considered a natural progression of DBS, has a significant advantage for compounds in the later stages of the drug development process with a long history of analyses conducted in plasma: the switch from liquid plasma to DPS would make bridging studies unnecessary because the data generated in plasma and in DPS are directly comparable.
Compared to DBS, DPS requires more complex handling that is similar to liquid plasma, but there is still a significant cost savings due to simplified shipment and storage of samples at room temperature.
DBS sampling is increasingly of interest to pharmaceutical companies that appreciate its ethical, safety, quality, and economical benefits.
Luca Ferrari ([email protected]) is head of the bioanalysis preclinical technologies division at Aptuit Verona.



