April 15, 2010 (Vol. 30, No. 8)
Five Animal Products Have Launched, and Late-Stage Human Trials Are Ongoing
The DNA vaccines field is definitely regaining the momentum that it had a few years ago, according to David Weiner, Ph.D., professor of pathology and laboratory medicine at the University of Pennsylvania and chair of the “DNA Vaccines” meeting held last month in New Orleans. “There are a lot of exciting breakthroughs, and the clinical data is much more encouraging.”
“DNA Vaccines,” sponsored by BioConferences International, a Mary Ann Liebert company, covered a range of noteworthy topics including: new approaches to electroporation, vaccine formulation, vector design, and recent clinical trials including a large-scale trial of an HIV/AIDS vaccine in Thailand.
In the HIV/AIDS trial described by Colonel Nelson Michael, M.D., Ph.D., of the Walter Reed Army Research Institute, the vaccine showed a modest (30%) but statistically significant protective effect over unvaccinated controls. “While a suitable HIV vaccine is quite a few years away, we’re in positive territory,” Dr. Weiner explained. “Getting from 30 to 60 percent effectiveness is a lot easier than getting from 0 to 30 percent. It is a big challenge, but several efficacy trials for HIV are quite encouraging.”
Matjaz Peterka, Ph.D., manager at BIA Separations, discussed the challenges of purifying plasmids,whose large size, negative charge, and varied levels of coiling and supercoiling present special challenges.
Plasmids represent less than 0.5% of the wet biomass, and endotoxins and other impurities must be completely removed. Because of their size, plasmids diffuse slowly through the solvent, and they may not be able to easily penetrate chromatographic beads if the pore size is too small. Since most chromatographic media is optimized for protein applications, viral particles and plasmids will tend to adhere to the outside, greatly reducing the absorptive capacity of the beads.
Monolithic columns, on the other hand, are better suited for plasmid separation, according to Dr. Peterka. With large flow-through channels (1.5 µm) and high surface accessibility they are useful for “mega-molecular” purification. The result is higher dynamic binding capacity, a flow-independent performance with lowered shear forces. “In our strategic planning, we focused on selective precipitation, anion exchange chromatography, and hydrophobic interaction chromatography,” he stated.
BIA Separations’ selective purification component employs CaCl2, and the separation of nucleic acids is accomplished through the use of a convection interaction media (CIM) DEAE-tube monolithic column, a weak anion exchanger. This column is used for extremely fast, highly efficient separations of large molecules such as proteins or DNA, as well as smaller molecules such as peptides.
It is followed by efficient separation of supercoiled and open circular pDNA on the CIM® C4 HLD-1 tube monolithic column. Following optimization, scale up of the process can continue using an 8,000 mL column, which will purify up to 48 g of plasmid DNA.
“The CIM DNA purification process removes over 99 percent of all major contaminants, and is fast and scalable,” said Dr. Peterka. “The economics are highly favorable, with high binding capacity and low buffer consumption, resulting in high productivity.”
Contract Manufacturing
“DNA vaccines offer a host of advantages over conventional vaccine technology,” noted Henry Hebel, Ph.D., COO of VGXI, a CMO. “Because they can be produced rapidly, they are ideal for combating emerging diseases. Moreover, they are safe to produce and administer, and do not constitute a threat to the production team.”
But as other participants in the conference noted, plasmid manufacturing poses a number of critical challenges. The E. coli host is renowned for the large quantities of endotoxins that it produces, and plasmids are notoriously hard to work with.
“Ease of manufacture” is a relative term, and what may be easy on the benchtop may be devilishly difficult when scale-up time arrives. The field is also subject to the “intellectual property thicket syndrome,” in which patents crowd out one another, raising expenses and confusing legal issues. “Plan early and build twice as much product as you think you’ll need,” Dr. Hebel advised.
The VGXI team searches for the most straightforward solution, weighing relative risks and benefits, while taking into account intellectual property. The purification procedure that it selected consists of anion exchange membranes, followed by hydrophilic interaction chromatography, which is then followed by thiophilic interaction chromatography for polishing.
“We consider cell lysis the critical step in the purification process,” said Dr. Hebel, “At this stage we avoid any procedure that could shear the plasmids. We also look for alternatives to traditional purification chromatography during the subsequent steps.”
The plasmid concentration step is another difficult issue, as mishandling can cause shearing and other destructive changes to the preparation. “As we optimize our protocol, we look to remove roadblocks,” he continued. “We need to justify the designation of contamination levels, degree of acceptable supercoiling, limits of cleaning, and the amount of plasmid DNA required.”

VGXI offers contract cGMP production for a large number of plasmid needs, including DNA vaccines.
Accelerating Commercialization
Althea Technologies offers services in plasmid design and production, and possesses core capabilities in biological process development, according to Magda Marquet, Ph.D., co-founder. This includes formulation and analytical development, as well as all phases of upstream and downstream processing and the final testing and IND filing from preclinical to commercial scale.
The company’s initial goal was to implement a scalable process adapted to clinical applications. Manufacturing of plasmid DNA is now well defined, and scale-up is achievable. “We have developed a high-cell density fermentation and purification process for plasmid-based DNA vaccines that yields 200–500 mg/L initially, and up to 2 grams/L following optimization,” Dr. Marquet stated. “This process has been used successfully on our multiple cGMP campaigns to produce lot sizes as large as 30 grams of plasmid DNA.”
The purification process development must take into account the problem of endotoxin removal. In conjunction with clients moving into Phase II trials, Althea has reportedly advanced its handling of the material, resulting in an additional log reduction in both endotoxin (

Althea Technologies has developed a high cell density fermentation and purification process for plasmid DNA products that reportedly provides plasmid yields in the range of 100–2,000 mg/L depending on plasmid size and bacterial strain.
Transient Expression Enhancers
James Williams, Ph.D., vp for R&D at Nature Technology, discussed his company’s development of antibiotic-free plasmids as vectors for DNA vaccines.
Antibiotic selection is a standard approach to manipulating vector growth and isolation, but it has the disadvantage of requiring continuing exposure to the antibiotic in order to retain the plasmid, which may be cost prohibitive when large-scale projects are required.
Moreover, regulatory agencies recommend elimination of antibiotic-resistance markers in gene-based therapeutic protocols. To deal with this ruling, Nature Technology has taken advantage of the SacB gene, which codes for the enzyme Levansucrase. The activity of this enzyme is lethal in the presence of sucrose for most gram-negative bacteria. By adding sucrose to the media and modifying the plasmid to encode a 150 bp antisense RNA that represses expression of a host strain chromosome-encoded SacB gene, it is possible to dampen expression of the SacB gene and select for retention of the plasmid, thus avoiding the use of antibiotics.
Nature Technology’s NTC8385 antibiotic-free gene medicine vector complies with guidance for plasmid size and composition and it provides increased antigen expression. The system uses a chimeric promoter, composed of a portion of the human T-cell lymphotropic virus type-1 and a CMV enhancer. This combination increases translation of transiently expressed RNA but does not increase integration of gene expression, which could have serious side effects for the host.
“The NTC antibiotic-free, gene medicine vectors increased transgene expression with no effect on integrated gene expression,” Dr. Williams said. “The end result of the adoption of our technology is improved manufacturing yields and increased expression of the gene of interest.”
Minimally Invasive Electroporation
A major impediment to successful DNA vaccination has been the failure to achieve sufficient uptake of the DNA by host tissue. This roadblock caused the collapse of a number of clinical trials a decade ago. The problems have now been resolved through improvements in transfection techniques, specifically electroporation. Inovio Biomedical has aggressively pursued development of a minimally invasive electroporation device, according to Kate Broderick, Ph.D., manager, R&D.
With the Inovio platform, DNA is delivered via a standard needle and syringe, electrodes are applied to the tissue of the host, and short pulses of electricity are delivered, causing channels to open briefly in the cell membranes, allowing entry of the vector carrying the genes for the antigen of choice. Whereas, earlier electroporation studies were conducted with muscle tissue as the target, extensive work has shown that the skin is a more attractive alternative tissue. Gene-expression levels, as well as immune responses using the minimally invasive dermal device yields potent cellular and humoral responses, Dr. Broderick said.
She described how a DNA vaccine delivered with Inovio’s minimally invasive electroporation instrument provided 100% protection in mice against a lethal influenza challenge. The same approach generated a greater than 1:40 hemagglutination inhibition antibody titer response in guinea pigs and macaques.
According to Dr. Broderick, “our results suggest that the minimally invasive dermal device may offer a safe and efficient method to administer DNA vaccinations in a prophylactic clinical setting.”
The Inovio electroporation technique behaves as an adjuvant and can induce significant immune responses, including both antibody and T cells, she explained. The procedure is tolerable to the patient without anesthetics against the delivery mechanism, so it can be employed for repeated administration.
In addition to electroporation, DNA vaccines may be delivered using a custom-built syringe, according to Matti Sallberg, Ph.D., at Karolinska Institute and one of the founders of Tripep.
The Tripep device consists of three to 10 needles in close proximity. The needles are bored with holes on the sides in such a way that they spray inward toward one another, thus the tissue is blasted and overloaded with DNA from all directions. The device is simple and improves DNA uptake in a larger animal, Dr. Sallberg said. It works with commercial syringes, is disposable, and can be mass produced.
Dr. Sallberg also discussed studies on a DNA vaccine for human HCV, referred to as ChronVac-C. Delivered by Inovio’s electroporation platform, it produced a transient reduction in the viral load that lasted for two to ten weeks.
The concept is directed toward activation of host T-cell response by presenting the viral antigens in a unique manner that activates CD4+ and CD8+ T cells. Using a gene gun to deliver to the skin, or injection or electroporation to the muscle cells, the vectors move from the injection site to the dendritic cells where they stimulate activation signals.
So far, the procedure has been used in a Phase I trial with encouraging results. The study tested the safety and the HCV-specific immune response. Working under the supposition that the T cells in chronic HCV infection are dysfunctional, the procedure is aimed at improving activation of T cells by the vaccination procedure.
“We feel the current status of the work is cautiously promising,” Dr. Sallberg stated. “There are many HCV-based vaccine approaches that are in the clinic, and some may have an immediately beneficial effect when combined with appropriate standards of care.”
DNA vaccine technology has made impressive strides in recent years as products come on the market, although, so far, only in the animal vaccine field. “There are already five different animal vaccine products available. We’re there with small animal DNA vaccine technology, and with a number of late-phase human trials in progress, we look forward to approval of a range of new human vaccines, including flu, HPV, and several cancer vaccines.”
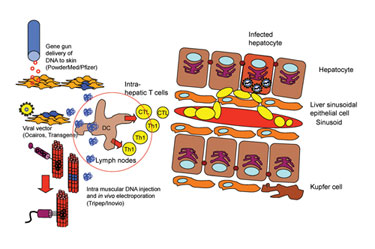
Scientists at the Karolinska Instititute and Tripep are working on a therapeutic vaccine for HCV.
K. John Morrow Jr., Ph.D. ([email protected]), is president of Newport Biotech and a contributing editor for GEN. Web: www.newportbiotech.com.



