June 1, 2009 (Vol. 29, No. 11)
Getting siRNA into the Right Place in the Cell Might Not Be a Problem for Much Longer
For about five years now, pharmaceutical companies have been interested in developing therapeutics based on the RNA interference (RNAi) pathway. Such drugs take the form of small interfering RNAs (siRNA), which perform the duty of interfering with expression of targeted messenger RNAs. And although the field has grown by leaps and bounds since its inception, there are still problems.
“The only real problem with therapeutic RNAi is getting the siRNA into the cell; every other problem is minutia in comparison,” says Steven Dowdy, Ph.D., investigator at The Howard Hughes Medical Institute, University of California at San Diego School of Medicine. A major reason for the challenges in RNAi delivery is that siRNA is a large (14,000 Daltons, on average), highly negatively charged molecule that will not enter cells unless they are artificially perturbed by chemical means.
Two of the most popular methods of siRNA delivery are nanoparticle- and liposome-based systems. The liposome-based systems have, up to now, been associated with liver-targeted pharmacokinetics, a phenomenon that restricts the therapeutic potential of RNAi to common diseases of the liver such as hepatitis, hepatocellular carcinoma, and hypercholesterolemia.
At CHI’s “RNA Interference Summit” to be held this month in San Francisco, various emerging and revised mechanisms of RNAi delivery will be presented. Dr. Dowdy will be one of the speakers at this meeting. “Our approach is an entirely different one than the rest in that it looks to have a different pharmacokinetic profile, so that we can potentially go after diseases in tissues other than the liver,” he says.
Dr. Dowdy’s approach involves the development of a fusion protein that contains a peptide transduction domain (PTD), an eight or ten amino acid, arginine-rich peptide that enables siRNA to be delivered to the entire population of cells in the human body. The delivery also occurs in a noncytotoxic manner, which already gives it a leg up on liposome- and nanoparticle-based delivery systems notorious for their cytotoxicity.
Essentially, because the PTD is arginine-rich the protein can interact with the highly negatively charged siRNA; when this occurs, they neutralize each other. The system is further enhanced with the binding of the siRNA by a double-stranded RNA-binding protein (DRBP), which further masks the highly negatively charged surface of the siRNA, thus allowing the entire complex to effectively interact with the target cell’s plasma membrane.
The complex is taken up into the cell by a specialized form of endocytosis called macropinocytosis that occurs in all cells. Once inside the macropinosome, the pH decreases from 7 to 5 and the DRBP, which is pH-sensitive, releases the siRNA into the lumen of the vesicle. There, the PTD causes a breach in the vesicle membrane, thereby releasing the naked siRNA into the cytoplasm, where it can cause a knockdown of its target through the RNAi pathway.
Dr. Dowdy will also present data on the effectiveness of this platform in over 30 cell types in culture, including many cancer cell types. “RNAi is the ultimate warhead to kill cancer cells because it has the ability to adapt as the genetics of the cancer adapt,” he says. “For personalized cancer treatment, the only putative drug on the table is RNA interference. Nothing will come close to this, if you could deliver it. If you can’t deliver it, then, obviously, there is no drug.”
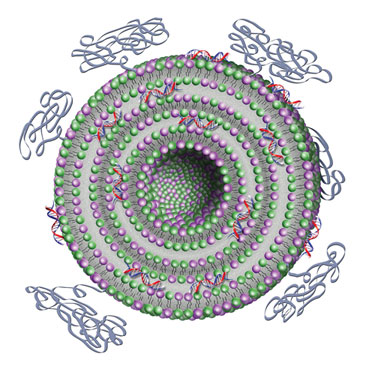
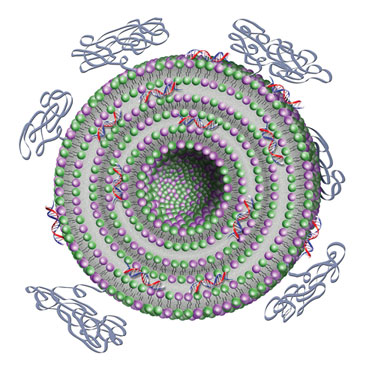
Silence Therapeutics’ AtuPLEX particles illustrate the liposomal moiety comprising the two lipids and the shielding properties by PEGylation.
Liposomes: Alive and Well
Although liposome-based delivery systems are fraught with cytotoxicity and liver-targeting effects, there are still innovative liposome-based delivery systems entering the field. Roger Adami, Ph.D., associate director of molecular pharmaceutics at MDRNA, will highlight new applications of the company’s DiLA2 platform for liposome-based siRNA delivery at the conference.
DiLA2 enables MDRNA to optimize liposome performance using modified amino acids, Dr. Adami reports. By varying the head-group, spacer, and alkylation of different amino acids, MDRNA is able to modulate the delivery properties of the DiLA2 in a liposomal formulation. Additionally, the DiLA2 platform can be combined with peptide-based nanoparticles that improve the efficiency of the delivered siRNAs.
Dr. Adami will show data on the knockdown of various biomarkers in the liver, such as ApoB, DGAT2, and PSKC9, in the BALB/c mouse model. In these experiments, low doses of siRNA designed to knockdown these endogenously expressed targets in the liver were administered intravascularly for systemic delivery.
MDRNA’s delivery platform, although liposome-based, can deliver siRNA to target organs other than the liver, Dr. Adami explains. As an example, he will show data on the use of this platform to deliver siRNAs to the urinary bladder through direct topical administration in a mouse model of orthotopic bladder cancer.
“We are showing delivery to different, therapeutically relevant organs and demonstrating that applying mixed modality delivery systems can achieve successful gene knockdown in vivo,” he adds. “I think the impact will be that people in the field who tend to feel that there are few options for successful delivery will see that there really are opportunities using combinations of different kinds of molecules.”
Another liposome-based RNAi delivery developer, Silence Therapeutics, will have two presentations at the conference. Angsar Santel, Ph.D., senior scientist, will talk about the use of the AtuPLEX technology—a nonencapsulating, liposome-based siRNA delivery platform—in the delivery of therapeutic siRNAs to the vascular endothelium. The experiments were performed as a proof of concept for inhibiting gene expression in vascular endothelium as a means for treating popular endothelial diseases.
Dr. Santel will present data from experiments designed to study the endothelial dysfunction that is common in bacterial infections of the lung. “We are interested in identifying genes that are specifically upregulated in vascular endothelium in the lung upon bacterial infection,” he says. He will also show that downregulation of an identified target gene after systemic administration of AtuPLEX can be exploited for a therapeutic application.
In the second presentation, Klaus Giese, Ph.D., CSO and vp of research, will show data on the firm’s lead RNAi therapeutic Atu027, an oncology drug that is scheduled to enter clinical trials this year. “From my point of view, it is clear that this is a novel treatment for angiogenesis, a therapeutic area that is rather novel in the field of RNA interference,” says Dr. Giese. “This will be the first program specifically designed to target tumor vasculature.”
Tekmira Pharmaceuticals develops RNAi-based therapeutics that utilize its SNALP technology, a lipid-based nanoparticle system for delivering RNAi-based therapeutics systemically. “Systemic delivery really appears to require an appropriately designed delivery system. And it seems that a lot of diseases will be best addressed by systemic delivery,” says Ian MacLachlan, Ph.D., CSO, who will present recent preclinical work on an RNAi-based therapeutic targeting ApoB in the liver.
Dr. MacLachlan will try to convey that Tekmira’s delivery technology is not only restricted to liver targets. “We have recently described not only the ability to deliver siRNA to the liver, but also the ability to extend the delivery to extrahepatic sites such as disseminated tumors,” he says. “The take-home message of my talk is that, through rational design of these lipid nanoparticles, we can design systems that enable safe and effective delivery of siRNA to different target tissues and for different indications.”
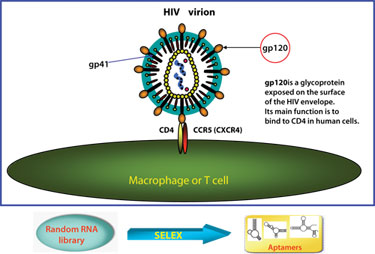
Aptamer targeting of the HIV-1 envelope glycoprotein gp120
(Beckman Research Institute)
Nonliposomal Methods
The use of aptamer-siRNAs will be covered by John Rossi, Ph.D., professor and chair, molecular biology, Beckman Research Institute. The power of the aptamer has increased by combining it with siRNAs to produce aptamers that are capable of selectively binding to human immunodeficiency virus type 1 (HIV-1) envelope protein, and ultimately blocking HIV-1 replication in cell culture.
The HIV-1 envelope was selected as a target because it is expressed on the surface of HIV-1-infected T cells and serves as a receptor for viral entry. Although data on these aptamers has been previously published, Dr. Rossi will present new data on how these aptamers have been shown to inhibit HIV-1 replication in several types of cell culture, including primary T cells.
He will also present an extension of the work in humanized mouse models for human stem cell engraftment. When a mouse model is treated with human stem cells, “the cells completely differentiate into all the lineages that HIV-1 infects in these mice, and in mice that have been treated with the aptamer we see complete suppression of HIV-1 replication, as compared to untreated controls, where replication is rampant,” says Dr. Rossi.
“We think this is an exciting approach for treatment of HIV-1 infection because it allows us to keep pace with the virus, which mutates pretty rapidly against siRNA. We can keep changing the aptamer and/or siRNA as the HIV-1 targets change.”
Johannes Fruehauf, M.D., Ph.D., vp of R&D at Cequent Pharmaceuticals, will talk about the company’s Transkingdom RNAi technology. Cequent focuses on the development of RNA-based therapeutics to treat human gastrointestinal disease. The company uses genetically engineered Escherichia coli cells to express, and then to deliver, therapeutic short hairpin RNA (shRNA).
This platform has been used to develop Cequent’s first clinical candidate therapeutic RNAi-based drug to treat Familial Adenomatous Polyposis (FAP). Patients with FAP have inherited a nonfunctional copy of the Adenomatous Polyposis C gene, which normally regulates expression levels of a key oncogene, beta-catenin, in intestinal epithelia.
The mutated form of the gene causes uncontrolled levels of beta-catenin, which leads to formation of cancerous polyps in the colon. The idea behind this new clinical candidate is to treat FAP patients with a therapeutic shRNA that can effectively keep the levels of beta-catenin at bay, and thus eliminate the possibility of polyp formation in those patients. Cenquent expects to begin clinical trials in the fall.
Development of RNAi-based therapeutics is relatively new to the pharmaceutical industry. Yet, in a short period of time, nearly all of the obstacles to the development of such drugs have been overcome. The remaining challenge is siRNA delivery. But with each advance, including the ones highlighted in this article, the pharmaceutical industry and academics alike will come closer to delivering RNAi-based drugs safely and on target.