March 15, 2014 (Vol. 34, No. 6)
Information-packed and bearing extraordinary diversity, glycans decorate more than 50% of mammalian secreted and cell surface proteins.
This fine architecture also presents challenges for their appropriate addition or reconstruction in engineered glycoproteins.
The recent meeting of the American Institute of Chemical Engineers in San Francisco featured presentations describing new advances in deciphering glycosylation reaction networks, optimizing biosimilar expression in Chinese hamster ovary (CHO) cells, and bioengineering plants and bacteria to serve as viable alternatives to traditional insect and mammalian expression systems.
Glycosylated proteins play critical structural and functional roles in a broad range of biological processes. These processes include protein folding, cell development, immunity, pathogen invasion, and cancer metastasis.
Glycosylation also regulates the pharmacokinetics and pharmacodynamics of bio-therapeutics. New developments in analytical tools, especially mass spectrometry, are generating vast amounts of data and deriving whole glycomes from cells and tissue.
Glycomics and Computer Modeling
At the State University of New York at Buffalo, scientists in the department of chemical and biological engineering have been developing computer algorithms for the study of these glycosylation processes. These scientists include Sriram Neelamegham, Ph.D., professor, and Gang Liu, Ph.D., research assistant professor.
Dr. Liu explained that while “the glycomes of many cells and tissues are now available in public databases, and such data are also being collected by independent investigators,” data analysis remains a challenge. “The quantitative determination of the underlying biosynthetic pathways that drive cell/tissue-specific glycomes is not possible without computer models. In particular, since glycomics experiments do not account for glycosyltransferase enzyme specificity and concentration data, it is difficult to derive a direct linkage between cell/tissue-specific enzyme activity and corresponding phenotype.”
To address this problem, the scientists developed an application package called GNAT (Glycosylation Network Analysis Toolbox). This program, which is freely available online, provides a streamlined approach for the construction, visualization, and simulation of glycosylation reaction networks.
Development of the application, explained Dr. Liu, involved three tasks: “First, we gathered glycan structure data from mass spectrometry experiments and enzyme specificity data using the IUBMB (International Union of Biochemistry and Molecular Biology), BRENDA (Comprehensive Enzyme Information System), and KEGG (Kyoto Encyclopedia of Genes and Genomes) databases. Second, we implemented a network construction algorithm by combining glycan structure and species-specific enzyme data. This allowed the synthesis of complete glycosylation reaction networks. Third, we developed ways to refine the model using the open-source toolbox.”
The team tested their models on two cell lines. “We assessed N-glycosylation in Chinese hamster ovary cells and its mutants,” recalled Dr. Liu. “We also assessed O-glycosylation networks in a human promyelocytic leukemia cell line, HL-60.”
“In both of these experiments, the toolbox was able to extract core pathways involved during glycosylation. Thus, it is possible to obtain greater insight from high-throughput mass spectrometry data using quantitative modeling. Such knowledge can aid the engineering of specific glycoforms on therapeutic proteins, and it can also enhance biomedical investigation of human diseases.”

At the State University of New York at Buffalo, glycosylation network analysis starts with the collection of glycomics, enzyme, and pathway data. This information is subsequently processed via network inference algorithms and quantitative modeling methods to reconstruct cellular glycosylation reaction networks.
Plant-Based Systems
Expression of appropriately glycosylated proteins in mammalian cells can be a challenging and expensive task. A less expensive alternative is the use of genetically manipulated plant-based systems. But can plant cells, with their different glycosylation machinery, decorate mammalian proteins properly?
A team of researchers at the University of California, Davis, are tackling this complex problem. Led by Raymond Rodriguez, Ph.D., a professor of molecular and cellular biology, and Karen A. McDonald, Ph.D., a professor of chemical engineering and materials science, the Davis team is carrying out an interdisciplinary program—combining plant science, chemistry, chemical engineering, and molecular biology—to achieve its goal of in vitro, post-production sialylation of an important human enzyme.
“We performed collaborative studies in which plant cells are used to express recombinant human butyrylcholinesterase (rhBChE), a serum-based bioscavenger for neurotoxic organophosphates (OP) like the deadly compound sarin,” said Dr. McDonald. “Current therapies are designed to elevate serum levels of BChE, but a single dose can cost as much as $10,000. We performed studies to express rhBChE in plants utilizing a novel means to create appropriate modifications in post-production.”
The Davis team utilized viral amplicon-based gene expression systems that compared rhBChE production via Tobacco mosaic virus versus Cucumber mosaic virus in Nicotiana benthamiana, a relative of tobacco and a commonly used model organism in plant research.
According to Dr. Rodriguez, “Development of rhBChE is a pressing national security issue. The U.S. government is seeking economical ways to mass produce this enzyme. However, BChE is a real challenge as it is a tetrameric protein with each monomer housing nine potential N-glycosylation sites. We targeted the protein to different subcellular compartments and found important differences in how the protein was glycosylated.”
Another novel aspect of the studies dealt with in vitro sialylation, the final “polishing” step for N-glycans. “Sialylation of glycoproteins is an end-stage modification that is critical for many processes such as maintaining stability, protein half-life, and immunogenic properties,” commented Xi Chen, Ph.D., professor of chemistry. “Since plants are incapable of sialylating glycoproteins, we employed post-production multistep enzymatic reactions to systematically add sialic acid to the termini of the N-glycans.”
Dr. McDonald noted that although the Davis team is in the early stages of bioengineering plant-based systems, the results are already encouraging. “We will continue to optimize these systems and also incorporate computational modeling of glycoproteins. Given that more than 30% of all commercial biopharmaceuticals are glycoproteins, our results suggest plant-based systems are viable alternatives to standard mammalian and insect expression systems.”
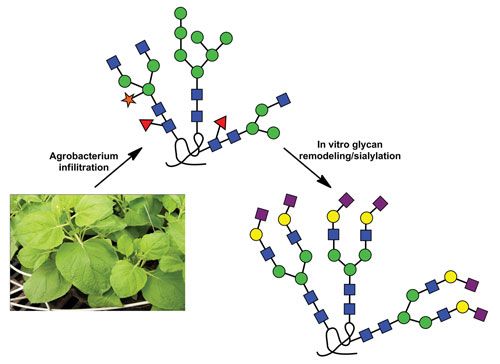
In vitro glycosylation strategy for plant-made glycoproteins [University of California/Davis]
Designer Glycosylation in E. coli
Many proteins including those of eukaryotic origin are expressed in bacterial systems such as Escherichia coli. However, bacteria cannot appropriately glycosylate eukaryotic proteins. To address this limitation, a team of researchers at Cornell University has engineered the first human-like glycosylation pathway in E. coli.
Matt DeLisa, Ph.D., professor of chemical and biomolecular engineering, led the team: “We pursued a ‘bottom-up’ glycoengineering approach that creates a synthetic glycosylation pathway for the biosynthesis of a precise glycan structure.”
These designer glycans were constructed by reprogramming E. coli with a series of five heterologous enzymes, which were predicted to produce appropriate glycan structures. “We can assemble diverse combinations of heterologous enzymes including glycosyltransferases (GTases) and oligosaccharyltransferases (OTases) to create glycans on demand, which can then be conjugated to a given biopharmaceutical product for tailoring its activity, stability, half-life, and immunogenicity.”
The group initially tested their strategy on two proteins, E. coli maltose-binding protein and an anti-β-galactosidase single-chain Fv antibody fragment. They characterized the products with a bevy of enzymatic tests as well as via MALDI-TOF/TOF and NMR analysis. According to Dr. DeLisa, “The results showed we had successfully generated the designed glycans and conjugated them site-specifically to eukaryotic proteins.”
A few challenges remain before the system is ready for prime time. “Endowing bacteria with specific glycosylation enzymes is not a magic bullet for making biologics as one still needs efficient protein expression and folding,” explained Dr. DeLisa. “Another challenge is the overall efficiency of glycosylation. However, we are continuing to develop new strategies and are making significant headway in understanding and manipulating the system.”
To address these challenges, the DeLisa lab is working to unify glycosylation in E. coli with advanced protein engineering tools such as by applying new ‘glyco-display’ technologies including cell surface display and phage display systems.
“Overall, engineering of defined glycosylation pathways in E. coli provides a new tool for the production of human therapeutics and vaccines that possess structurally complex glycans,” concluded Dr. DeLisa. “The landscape continues to change and as we continue to make improvements, we expect E. coli will take its place alongside other eukaryotic expression platforms as a viable system for glycoprotein biosynthesis.”

Matt DeLisa, Ph.D. (right), a professor of chemical and biomolecular engineering at Cornell University, meets with postdoctoral fellow Jack Huang to discuss strategies for engineering bacteria into microfactories that create therapeutic proteins.
Biosimilars in CHO Cells
Developing biosimilars (generic biologics) requires a comprehensive understanding of the expression and production systems utilized. This can be time consuming and expensive. A team of researchers at the University of Alabama are studying the mechanisms that rationally regulate such processes using three CHO cell lines.
The team’s lead scientist, Xiaoguang “Margaret” Liu, Ph.D., assistant professor of chemical and biological engineering, stated, “The targets of this study are to investigate various CHO expression systems using omics technologies, develop an in-depth understanding of CHO-based protein expression, and create a platform to rationally express the interested biopharmaceuticals with the shortest timeframe.”
The team studied CHO K1, the parental line; CHO DG44, a slower growing metabolically engineered line with deleted dihydrofolate reductase gene (dhfr); and CHO S, an adapted faster growing line. “These cell lines can host a wide range of heterogeneous genes and regulate post-translational modifications (such as glycosylation, sialylation, and fucosylation) to improve the bioactivity, extend shelf life, and reduce the immunology response of the expressed therapeutic protein.”
First, the scientists grew the suspension cells in chemically defined media suitable for each. Second, they conducted proteomics analysis of host cells. Third, they expressed biosimiar immunoglobulin (IgG1) and recombinant erythropoietin (EPO), compared glycosylation patterns, and investigated host cell regulators.
“Our studies demonstrate that the commercial basal media—that is, CD M4CHO (CHO K1), EX-CELL (CD DG44) or CD FortiCHO (CD DG44 and CHO S), and CD CHO (CHO S)—are good starting points to achieve high cell density and protein production,” explained Dr. Liu. “However, the specific nutrients need to be developed using metabolomics and metabolic flux analysis (MFA).”
The proteomics profiles of 1,308 host proteins were investigated and compared. Some cell regulators of biopharmaceutical glycosylation, stability, and cell growth were identified. These included heat-shock proteins, glucose-regulated proteins, proteasome, hypoxia-regulated protein, transglutaminase, and disulfide-isomerase.
Dr. Liu predicted that a better understanding of host cell regulation and protein glycosylation will provide a powerful tool to rationally design bioprocessing of both innovative biopharmaceuticals and generic biologics. However, challenges remain. “It is desired to develop an efficient strategy to analyze the big omics data,” concluded Dr. Liu. “To solve this issue, we plan to develop genome-scale models integrating CHOnomics profiling to direct the rational development of an effective bioprocessing platform.”
Constancy of Batch Production
The use of microbioreactors in producing monoclonal antibodies in mammalian cells requires that conditions be defined with exactness. Such exactness is important in general, for it provides consistency, and it is particularly important for N-glycosylation.
Scientists at the Institute for Chemical and Bioengineering, ETH Zürich have devised a way to couple experimental data with mathematical modeling to optimize glycosylation.
According to Miroslav Soos, Ph.D., senior scientist, “We performed experiments to assess monoclonal antibody production with different operating parameters and various feeding strategies in a high-throughput microreactor that can run a number of conditions in parallel. We examined conditions known to have a significant impact on N-glycosylation including pH, trace elements, carbon sources, and byproducts such as ammonia.”
Dr. Soos’ team measured basic metabolites and intracellular nucleotide sugars to create a mathematical model of quality attributes based on the dynamics of N-linked glycosylation. The team’s analysis, said Dr. Soos, provides a means to optimize growth conditions most favorable to appropriate N-glycosylation.
“The time to start and optimize cell growth and N-glycan parameters is at the beginning of the project, since we cannot modify it with downstream purification later,” explained Dr. Soos, who added that a combined experimental and modeling approach could provide a quality-by-design instrument during bioprocess development as well as define parameters for large-scale production of monoclonal antibodies.
As the field of glycobiology continues to advance, not only will its myriad biological roles be better understood, but its utility in therapeutics will remain a mainstream focus.



