March 15, 2017 (Vol. 37, No. 6)
In Flow Cytometry, “Fast” Is About Speedily Collecting and Analyzing Data
Technological change is often driven by the needs of people. Flow cytometry is no different. The designs of instruments and processing techniques are evolving to meet the needs of researchers and drug developers. The aim is to allow faster, more accurate experiments.
Instrument manufacturers and scientists gathered in Leeds last year for flowcytometryUK, to discuss developments such as the introduction of new immune biomarkers, innovative dyes and panel designs, and improved techniques for detecting rare events.
One market for innovations in flow cytometry is global pharmaceutical research devoted to immunotherapy drugs, such as therapeutic antibodies. One of the challenges of developing these drugs is that regulatory agencies want to understand the exact structure of the antibody.
Improving Targeted Labeling
Labeling a therapeutic antibody for flow cytometry can alter its structure and compromise its function. Thermo Fisher Scientific now offers a labeling technology (SiteClick™ Antibody Labeling System) that consistently attaches to a known site on the antibody. The label attaches to the Fc domain, avoiding the antibody’s binding site and preserving its function.
“We’ve offered something similar in the past,” said Chris Langsdorf, Ph.D., staff scientist of protein and cell biology at Thermo Fisher Scientific. “The advance here is a wider range of dyes and tags.”
During his talk, Dr. Langsdorf discussed two well-known models of therapeutic antibodies—Rituxan (rituximab) and Herceptin (trastuzumab). In an interview with GEN, he explained that SiteClick labeling is also compatible with antibodies that incorporate unnatural amino acids. The label can attach to binding sites engineered into the antibody.
Another growing innovation in drug development is antibody-drug conjugates (ADCs). These use a monoclonal antibody to target cancer cells, and they can deliver a payload of potent cytotoxic drugs. Thermo Fisher Scientific has developed a line of fluorescent (pHrodo®) dyes in red and green, which fluoresce only when they’re taken into a lysosome or endosome—acidic compartments inside a cell.
“The pH-sensitive dyes are second-generation constructs,” noted Dr. Langsdorf. “The novelty here is they’ve been redeveloped to be ideally suitable for antibody labeling.”
Adding More Parameters
Flow cytometry experiments that look at large numbers of parameters are becoming more common. One driver is the need to look at multiple biomarkers simultaneously—a need, observes Barry Moran, Ph.D., head of flow cytometry at Trinity College Dublin, that is especially marked among immunologists.
Dr. Moran’s research focuses on the rare but severe skin disease Hidradenitis suppurativa. Previous studies had found it hard to identify the immune cells involved in the disease and the inflammatory signals expressed, so Dr. Moran turned to flow cytometry.
“There’s a limited sample size, and a small number of immune cells in that sample,” he pointed out. “So the best way to glean the most data is high-parameter flow cytometry, where we use lots of fluorescent colors at once.”
Dr. Moran used 15 fluorescent markers for the study presented in his talk. He wanted to identify the various cell populations and their signatures. He stressed that in his work, it isn’t enough to look at one or two cytokines, for example. What is more relevant is the balance of pro- and anti-inflammatory cytokines being simultaneously expressed by each cell.
“Large immunophenotyping studies are definitely an emerging area of investigation,” stated Lorinda Turner, Ph.D., postdoctoral research associate at the Department of Medicine, University of Cambridge. According to Dr. Turner, this development is attributable, in large part, to the growth in the number of fluorochrome conjugates available for labeling particles of interest, and the introduction of flow cytometers with more lasers and filters for detection.
“These multiparameter machines are becoming more and more common across the world, leading to an exponential increase in these higher-dimensional immunophenotyping studies,” Dr. Turner insisted.
The NIHR Cambridge BRC Cell Phenotyping Hub, a collaborator on Dr. Turner’s project, has recently acquired a BD Biosciences Fortessa X30 (FACSymphony). This is a high-end machine with the capacity to detect fluorescent signals in 28 different channels, with the option to upgrade to 50 channels.
Dr. Turner’s work is the first to use complex, multiparameter flow cytometry to investigate the immune system in schizophrenia. The idea that the immune system may play a role in the development or pathology of schizophrenia and other mental health disorders has only become an intense area of research in the last five years or so, she says.
Her work aims to identify variations in the immune cell populations in various psychiatric disorders, including schizophrenia. By identifying alterations in the proportion or activation of these immune cells, they can be developed as biomarkers or therapeutic targets.
Dr. Turner indicates that the major challenge of her research is making sense of the high-dimensional flow cytometry data. In addition to recording cell counts, investigators must measure multiple cytokines, perform gene-expression analysis, and analyze complex clinical data.
To tackle the data challenges inherent in her work, Dr. Turner uses the multivariate statistical technique of partial least-squares regression to reduce the dimensionality of the data. This allows the identification of a few key combinations of immune-cell phenotypes predictive of disease.
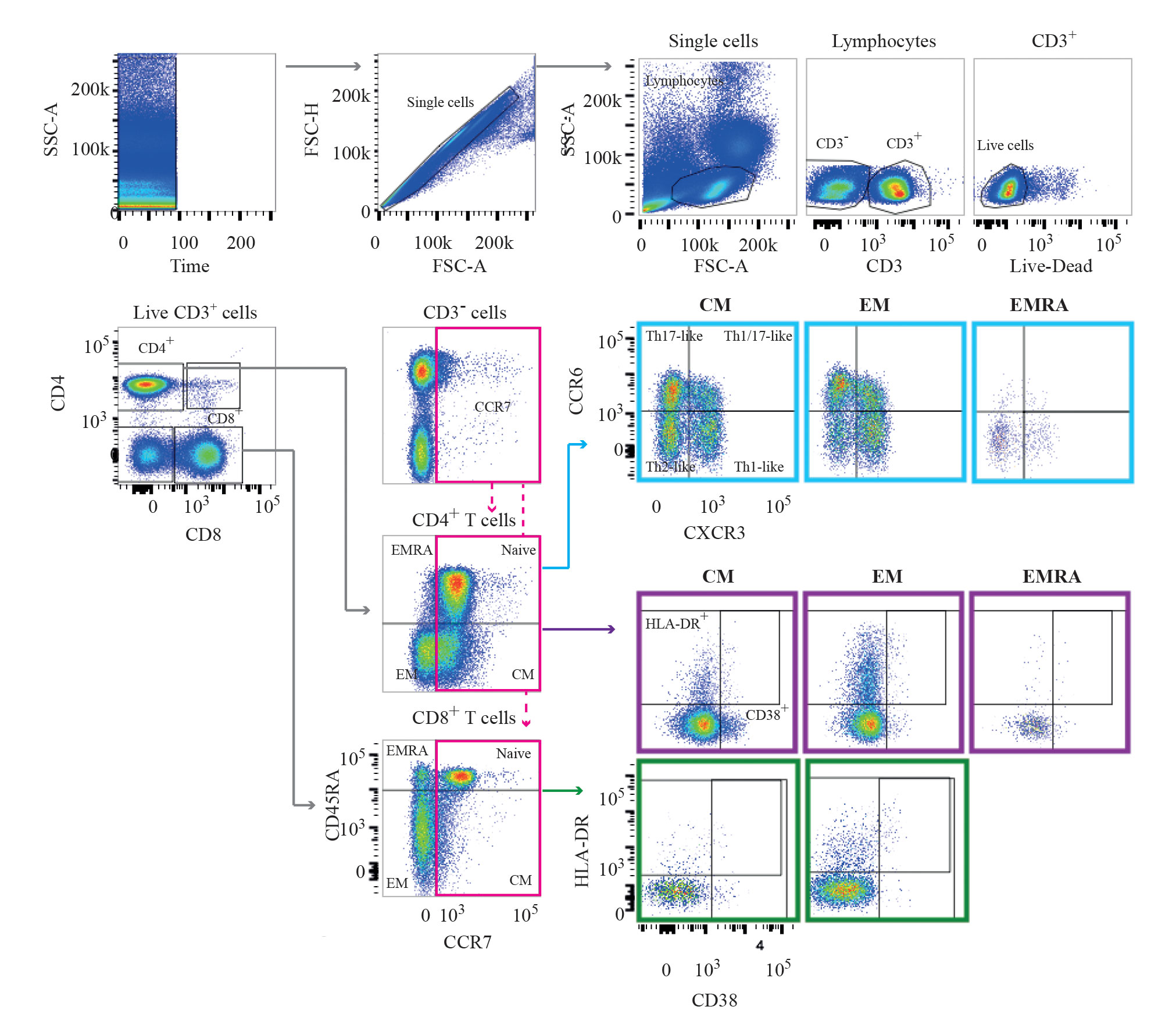
In flow cytometry analysis, gates and regions are placed around populations of cells that have common characteristics with respect to properties such as light scattering and biomarker expression. This image, from the University of Cambridge, shows the results of a gating strategy that was used to define T-cell subpopulations.
Reducing Spillover
One of the challenges to multiparameter flow cytometry is spillover, where the emissions spectra of fluorescent dyes overlap, and emissions from one dye are detected at a detector designed for another. This creates optical interference that must be compensated for.
With Dr. Moran’s study, particularly, some cells were coexpressing several biomarkers of interest. The risk of spillover was high. Optimizing the panel of fluorochromes to reduce spillover was an important part of the study.
One solution is improving fluorescent dyes so they are less likely to cause spillover when excited by laser light. Thermo Fisher Scientific is releasing new Super Bright violet dyes that, according to Dr. Langsdorf, have a narrow emissions spectrum to reduce the risk of overlapping signals from different fluorochromes.
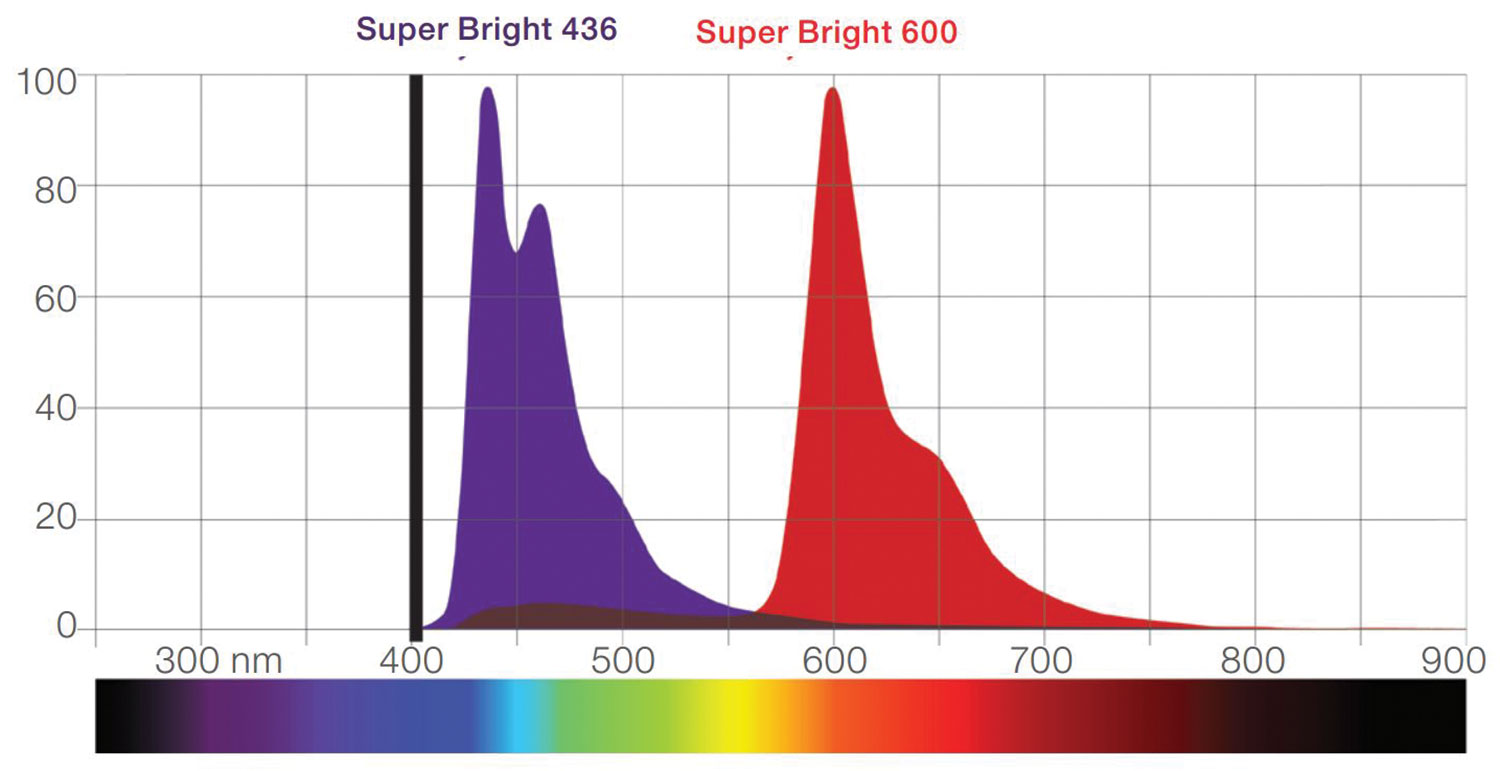
Thermo Fisher Scientific has developed Super Bright polymer dyes, which are violet laser-excitable fluorophores. They are designed to enhance multiparametric flow cytometry analysis by increasing the number of markers off the violet channel. The dyes, given the characteristics of their emission spectra, allow for better discrimination of dim populations and reduce nonspecific interactions.
Expediting Analysis
Another trend is the speeding up of flow cytometry to improve rare-event analysis. Dan Fox, director of R&D at Propel Labs, worked to develop the new Bio-Rad ZE5 Cell Analyzer. He worked with a panel of 10 customers to build the cytometer to their specifications.
One of the features is an integrated plate loader, which claims to process a 96-well plate with less than 0.5% carryover between samples in less than 15 minutes. According to Fox, this was developed in response to customer feedback about plate loaders sitting on shelves. The customers complained that manually performing tasks such as maintenance, cleaning, and connecting the loader to the flow cytometer was too laborious.
“They had 60 to 80 tubes, with multiple colors and patient samples,” Fox confided. “They wanted to get through these quickly rather than one tube at a time.”
The new plate loader has an automated wash station, which follows along with the probe of the sampler. This injects a cleaning series of liquid and bubbles that washes the internal line between samples. According to Fox, this saves time from a conventional cytometer, which pauses to wash itself between samples.
One benefit of the fast loader is that customers can process more cells, obtain information more quickly, and generate better statistics, explains Fox. The system also has custom lasers and a high fluid pressure of 10 psi that, according to Fox, allows it to process up to 100,000 events per second. This was developed for customers looking for rare events, such as circulating tumor cells.
“People have been hampered by how long it takes to do an experiment,” commented Fox. “If they had been looking for a one-in-a-million or one-in-a-billion event, that experiment would have taken six to eight hours.”

The Bio-Rad ZE5 Cell Analyzer is a flow cytometer designed for speed and flexibility. It integrates a universal plate loader that can accept single tubes, tube racks, or 96- and 384-well plates, and it operates with up to five lasers and 30 detectors. In this image, a scientist is changing the instrument’s internal fluid containers. The plate loader is behind the green-tinted glass panel.
Expanding Detection Range
Scientists are gradually increasing the range of molecules that can be detected by flow cytometry. Detecting mRNA, in conjunction with counting CD4 cells, has applications in monitoring viral load in HIV patients. However, mRNA occurs in small quantities, and signal amplification techniques such as PCR tend to destroy the cells, making them unusable for flow cytometry.
“By the time you’ve got to 93°C and back down to 50°C again, 20 or 30 times, it’s not practical to put the cells through a flow cytometer,” said Paul Wallace, Ph.D., professor of oncology and director of the department of flow and image cytometry at the Roswell Park Cancer Institute.
He has refined an amplification technique for mRNA that works at lower temperatures and is gentler on cells. The Branched DNA technique is a multistep process that can produce a theoretical 8,000- to 16,000-fold amplification in the fluorescent signal measured by a cytometer.
“A cell can be producing a lot of copies of mRNA or few copies,” Dr. Wallace explained. “We’re able to detect as few as five copies with flow cytometry.”
The Branched DNA technique uses “Z-Probes,” each containing 20 complementary DNA bases, to bind to the targeted mRNA sequence. A preamplifier then binds to the Z-probes, amplifier molecules bind to multiple sites on the preamplifier, and fluorescent labels bind to multiple sites on each amplifier. Each step in the process amplifies the target signal around 20-fold.
The technique can also be used to study the kinetics of protein expression inside cells. For example, it can monitor the time between a cell beginning to produce an mRNA and its expression as a protein. According to Dr. Wallace, it’s also an alternative to a standard antibody approach to cell labeling: “If there’s an antigen we’re interested in, but we don’t have an antibody, we can use the Branched DNA method as an alternative approach to determine if a gene is expressed by the cell.”



