February 1, 2011 (Vol. 31, No. 3)
Cell Quality, Growth Environment, and Analytical Capability Upgrades Are All Being Pursued by Companies
With an estimated 90% of lead candidates from in vitro screens ending up as also-rans—despite the spiraling costs of development—new ways of eliminating these candidates earlier in the drug discovery process need to be developed. A major theme running through Visiongain’s recent London-based “Cell Based Assays” conference is that while current tests are good at picking up blatant red flags such as carcinogenesis and necrosis, they can be nearly colorblind to the shades of pink represented by heart arrhythmias and the accumulation of toxic metabolites, for example.
“The holy grail of early-stage drug discovery is the subtleties in and around toxicity that really kills programs—it’s not overt cell death,” noted Quin Wills, CSO and co-founder of SimuGen.
The screens for more subtle toxicities—where they exist—tend to be more time- and labor-intensive, and are often relegated to use on later-stage candidates in which substantial resources have already been invested. “In pharma there is a great desire to move to assays using primary cultured cells farther upstream in screening cascades,” said Steven Shamah, director of cell biology for SRU Biosystems. Thus, there is a need for high(er)-throughput cell-based methods that can say more than just that the cell is dead.
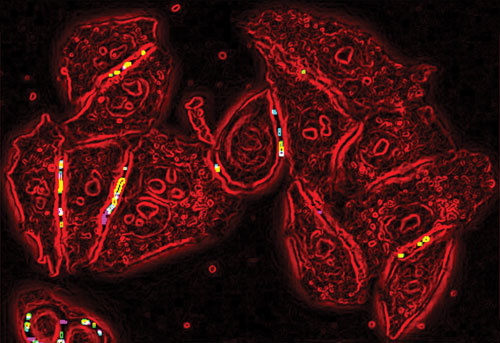
In high-content screens, very high resolution and fluorescence imaging can capture detailed cell phenotypes (i.e., organelle health and expression of genes and proteins), all at a single-cell level. According to SimuGen, single-cell methods are increasing in importance as they allow researchers to accurately capture heterogeneity.
Billions and Billions of Cells
Sometimes the bottleneck in cell-based screening is the availability of the high-quality cells themselves. “Even when pharma does have the opportunity to use human primary cells, they end up with cells that are of highly variable composition, quality, from different donors at different ages, with different underlying morbidity, environmental influences, postmortem intervals, and a number of variables that they can’t control,” pointed out Stephen Minger, GE Healthcare’s global director of R&D for cell technologies.
To address this issue, GE Healthcare acquired the rights to develop Geron’s (www.geron.com) human embryonic stem (HES) cells for the nonclinical research market. HES cells are highly stable and can be grown to unlimited numbers in culture. “They represent a tremendously valuable source of cells where you can provide a consistent quality of cells with a consistent genetic background, week-in, week-out,” Minger says, “that could, in principle, give rise to every type that pharma might want to use for their safety studies or even in primary drug discovery.”
Minger and his team differentiate industrial-scale quantities of HES cells into highly functional populations of human cardiomyocytes, which have been characterized developmentally, morphologically, biochemically, and functionally, and have been validated both internally and externally. The cells were screened against large panels of compounds that are known to interfere with action potentials, and were found to be equal to or better than current FDA-mandated safety tests. “In some cases,” he added, “they’re predictive when the standard animal tests are not in picking out long QT [delayed repolarization].”
GE Healthcare is developing a range of assays that, when combined with their capabilities in high-content imaging, can look at both the population and individual cell level for compounds that interfere with both electrophysiological properties and with subcellular organelles like the mitochondria and membrane integrity. Ultimately, they are trying to develop a whole range of cell types—HES-derived hepatocytes are already in the pipeline—with broad genetic diversity gained from utilizing cells from national banks. “Now, all of a sudden, you almost have a Phase I in a dish.”

GE Healthcare cardiomyocytes, derived from NIH-approved human embryonic stem (hES) cells, in a live multiplexed toxicity assay are stained with probes that report nuclear status (Hoechst 33342, blue), mitochondrial membrane potential (TMRM, red), calcium mobilization (Fluo-4, green), and plasma membrane integrity (TOTO-3, not shown).
Added Dimensions
Animal physiology is about more than just isolated cells—biological systems work as multicellular units of tissues and organs that sense their mechanical microenvironment. Cells allowed to grow on soft, three-dimensional scaffolds will mimic the tissue better than those growing on a hard, two-dimensional tissue culture plates, postulated biophysicist Tetsuro Wakatsuki, co-founder and CSO of InvivoSciences.
InvivoSciences grows isolated primary cells or differentiated stem cells in specialized 8- or 96-chamber systems that can be read on a microplate reader. It uses mainly a collagen base, “but we mix in a lot of other factors and matrix,” Wakatsuki said. For cardiac assays, because the natural cardiac tissue is composed of more than just muscle cells, “we use different cell types to be able to mimic the cardiac tissue.” Such cultures have the added benefit of being viable for months rather than the weeks a 2-D culture will typically last.
The system is used to measure the effect of compounds on contractility and metabolic activity. Yet some drugs function through idiopathic pathways—through unknown mechanisms. “So, in order to detect the adverse effect of the drugs, you need to have a functional tissue” with, for example, a representative biomechanical structure. “That’s what we provide.”
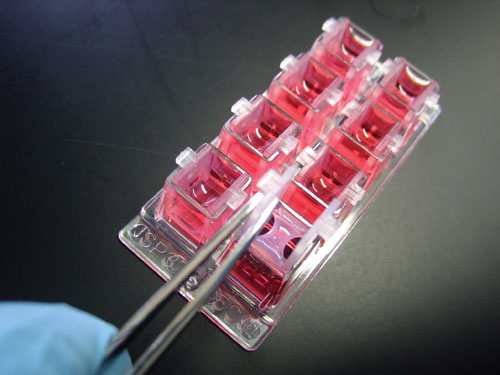
InvivoSciences’ IVS Inserts allow the growth of 3-D tissue constructs in a standard 8-well chambered coverglass. Suspended tissues form across the support bars allowing measurement of mechanical contractility and stiffness using the Palpator tissue mechanics analyzer. Tissues in culture can be read on plate readers and observed under a microscope through the coverglass bottom. The removable tissues can also be used in tissue construct implantation studies.
Metabolic Spots
“You would like to incorporate not only basic screens of a cell that’s in contact with a potentially toxic compound, but also be in a position to mimic what the body does,” pointed out Jonathan Dordick, director of the Center for Biotechnology & Interdisciplinary Studies at Rensselaer Polytechnic Institute and co-founder of Solidus Biosciences. When a drug goes into the body it is metabolized by the liver, “so you’ve got to take into account metabolism as well.”
The cornerstone of Solidus’ platform is the data analysis toxicity assay chip (DataChip), a microarray slide capable of supporting 1,080 3-D cell cultures embedded in alginate. Test compounds are added to a second slide, the metabolizing enzyme toxicity assay chip (MetaChip), which contains spots of enzymes such as liver P450 isoforms that catalyze reactions. When the two chips are sandwiched together, the parent compounds and their metabolites can “then transfer down to the DataChip and enter into the alginate spot, enter into the cells, and do what they do,” Dordick explained.
The chips are separated after a period of time—up to 24 hours—and the DataChip is then allowed to incubate in cell culture medium for another couple of days. The DataChip is washed, stained, and read on a typical microarray scanner. “The number of cells correlates to a dose-response as a function of concentration of drug that you added.”
Solidus is currently developing a second-generation platform. Here, the DataChip will physically snap on to a microwell containing the MetaChip, making it easier for customers to work with in-house, Dordick noted.
Cell and Substrate
Heisenberg’s uncertainty principle states that the very process of measuring something necessarily alters it. The biological corollary is perhaps that the less manipulation done to a system, the more likely a cell-based assay will reflect what goes on in vivo.
SRU Biosystems uses its label-free BIND technology to detect the interaction between cell and substrate. “Any contact the cell has on the sensor surface, any increase in cell adhesion with the sensor surface, causes the wavelength of light that reflects back off the surface of the sensor to shift in the positive direction,” explained Shamah. “We measure that shift.”
Cells either change their adhesion or undergo morphological changes as “responses to a variety of different classes of receptors and stimuli, like G-protein-coupled receptors, receptor tyrosine kinases, things that activate certain classes of ion channels, just to name a few.”
The BIND Reader allows for high-throughput snapshots of an entire 96-, 384-, or 1,536-well plate—a 384-well plate can be read in 15 seconds. And because cells are not modified or perturbed by labeling, Shamah noted, it can also document real-time kinetic responses to stimuli as well.
SRU Biosystems plans to introduce the BIND Scanner, capable of imaging at a resolution of about 4 microns per pixel, in Q1. It allows cells plated at low densities, or in mixed populations, to be followed individually as they respond to different stimuli.
Online Analysis
For those focused more on the visual, Wimasis offers online analysis of optical cell-based assays without the need to buy hardware or install software. “We provide image-analysis algorithms that are available through a web-based analysis system,” says CEO and co-founder Kilian Schramm. “You receive the analysis results automatically, without the need of parameterization or adaptation to your specific needs, enabling an objective comparison through your different lab hardware setups.”
Wimasis currently offers analyses in several different areas, including angiogenesis and cell migration, and is developing others in apoptosis, autophagy, and chemotaxis. Wound-healing parameters, for example, include currently cell covered area, speed of closure, and acceleration characteristics determined from a time-lapse image series.
The company also works with researchers, companies, and institutions to customize algorithms to their specific needs. Suppose there’s a multisite clinical trial, for example. “We develop an intuitive analysis setup that can detect what needs to be detected and we develop a metric system that quantifies those objects correctly—maybe in size, in shape, in amount of pointy tube-like edges, which indicates a metastasis building, and so on,” Schramm explained. “Basically, anything in the image you can see we can quantify as well.”
Wimasis’ cloud-computing platform utilizes a fully scalable architecture in parallel, allowing it to handle a nearly unlimited number of images in almost any still photo format, Schramm said, nearly in real time. He expects the system to be able to accept actual movie formats beginning in Q2, 2011.
Decision Analytics
Once the data is generated and crunched, then what? Can we accurately rank our compounds? Do we have confidence in that ranking?
SimuGen’s raison d’etre is to include a third stage after biological modeling and data modeling—which Wills calls “decision modeling.” Its web-based algorithms are designed to be agnostic: To seamlessly combine into a single model gene and protein-expression data, cell viability and impedance assays, and high-resolution microscopic imagery, for example, “in an easy way that everybody can understand, and in a way that helps us make a decision,” he explained.
Researchers can implement their own model, utilizing their favorite cell types, assays, and endpoints. The software allows researchers to combine those into a screen that can be sold or used in-house.
Others may want to utilize SimuGen’s own models, currently focused on six major liver toxicity endpoints. “Liver is by a long stretch the biggest reason for drug failure on the market,” noted Wills. Experiments can be performed in-house, or by the company’s service partners, and then analyzed for six different endpoints.
The software will perform quality control and will rank the chemicals, showing which toxicities are occurring and at what concentration toxicity begins to occur, he explained. “It will then also show you at a global level how all the chemicals are looking relative to each other, so that you can start spotting patterns of chemicals that are behaving similarly in terms of their toxic profiles.”
The latest version of SimuGen’s software, Wills said, includes the ability to weight models—for example, downgrading certain endpoints relative to others. “Phospholipidosis should be far less of a flag compared with carcinogenesis.” This version also allows thresholds to be included. If blood levels of a compound aren’t likely to be seen at greater than 10 micromolars, “as a rule any toxicity we pick up at 100 micromolars is really irrelevant.”



