September 1, 2013 (Vol. 33, No. 15)
A Closed System Can Reduce the Number of Open Events and Improve Sterility
Current potential bioprocess solutions for the scaleup of adherent cells include cell stacks, robotic flask handling, packed-bed culture, hollow-fiber bioreactors, and microcarriers or aggregate culture in stirred tanks.
Cell stacks and robotic flask handling can increase the total surface area in culture but do not remove the open events (e.g., taking the top off a T-flask and exposing it to potential contamination) from the process or eliminate the wasteful feeding strategies inherent to flask-based systems. Packed-bed systems have traditionally been used for protein and antibody production and are not designed for the efficient recovery of cells.
The growth of cells on microcarriers can result in mechanical damage to the cells and difficulty removing the microcarriers from the cell product. Maintaining the proper aggregate size and cell viability in stirred tank bioreactors can require the use of enzymatic or mechanical methods that can damage the cells.
Automation has been defined as a handoff of tedious and routine tasks to more efficient and reliable machines. Progress in bioprocess automation has been slow because many of the current instruments are not up to the task.
Automation of a flask-based manual cell-culture process can solve many of the problems of scaleup. A closed system reduces the number of open events and improves sterility. Automation can reduce labor costs and improve throughput. Improved product quality and reduced variability can lead to better regulatory compliance and contain cost.
Automation of process steps eliminates human inconsistencies and errors while maintaining optimized culture conditions across multiple manufacturing sites. Finally, automation can aid in regulatory compliance and improve product comparability by locking in process changes.
Automated Hollow-Fiber Bioreactor
Terumo BCT’s Quantum® cell expansion system is an automated hollow-fiber bioreactor designed for the culture of adherent cells. The closed system can automate many of the tasks associated with flask-based culture, including cell seeding, reagent addition, feeding, and harvest.
Extensive work in our laboratory and the laboratories of others have shown that cells grown in the system meet or exceed the International Society for Cell Therapy standards. Mesenchymal stem cells grown displayed identical or improved morphology, phenotype, differentiation capacity, and karyotype (when compared to cells cultured on tissue culture treated polystyrene.
The largest change one can achieve with the system is the elimination of the handling (and open events) of numerous flasks required for the production of millions to billions of cells.
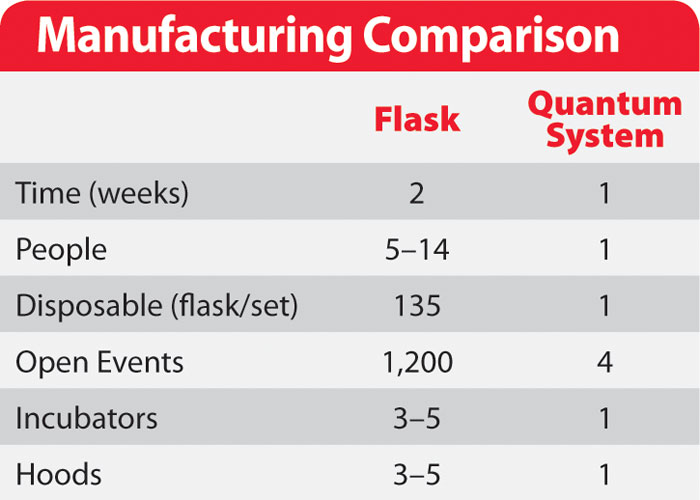
For example, a flask-based system for the production of two billion fibroblast cells would require the handling of over one hundred flasks, two weeks of culture time, and multiple operators. The same number of cells produced in a Quantum system would take just six days and could be managed by an individual working part time (Table 1).

Table 1. Manufacturing comparison of a flask-based system and the Quantum system for the production of two billion cells.
The instrument uses a number of predefined tasks to replicate the cell culture process from cell seeding to harvest. Each task can be customized to match the needs of a particular cell type. The system is controlled by a graphical user interface. Once optimized, a process can be locked to improve reproducibility and decrease run-to-run variability.
Additionally, a variety of reports can be generated for each run to meet current Good Manufacturing Practices regulatory requirements. The system also has remote alarm capabilities.
The perfusion-based feeding strategy can control the feed rate of the system from 0.1 mL/min to many mL/min. Gas exchange and temperature are controlled by the system. Metabolite concentrations can be measured at separate sampling ports for both the intracapillary and extracapillary loops.
This control of the perfusion feeding system can minimize the use of expensive media and improve cell quality by reducing cell stress resulting from excessive metabolite concentrations. A diagram depicting the control of metabolites including glucose and lactate in the Quantum system versus a typical flask-based or batch-fed process is shown in Figure 1.

Figure 1. Comparison of metabolite variation in the Quantum system compared to a flask-based system over nine days of growth.
The surface area of the hollow-fiber bioreactor is 2.1 square meters or approximately equal to that of a 40 layer cell stack. An individual device is capable of producing more than two billion cells depending upon cell type. Scaleup to larger lot sizes is possible because of the increased reproducibility and reduced manpower required to run multiple systems.
A skilled operator can manage up to 10 devices simultaneously. That translates to a lot size of over 20 square meters and over 20 billion cells per lot. The reduced footprint and ease of use of the system translates to an increased ability to meet the scale-up requirements of cell therapy manufacturing.
For example: a cell therapy requiring the rather low dose of five million cells per dose (Table 2). The system could produce 400 doses per device per lot. For a therapeutic indication present in two percent of the U.S. population over the age of 65, more than 2,000 lots would be required to reach all the patients. A laboratory with four full-time employees and 40 systems could produce all of the cells required for an entire year.
Adoption of the Quantum system is simplified by the use of predefined tasks for each step of the culture process. Each run can be fully documented for regulatory purposes by the generation of electronic records, user authentication, and remote alarming capabilities. Feeding and waste removal are precisely controlled by the perfusion system of the hollow fibers eliminating the big swings in nutrients and waste products common to batch-fed and flask-based cultures.

Table 2. Manufacturing requirements to reach a patient population as determined by lot size.
James Beltzer, Ph.D. ([email protected]), is senior cell processing specialist at Terumo BCT.