Clinicians are starting to become aware of the advantages of liquid biopsy, but they still have reservations. The point is, clinicians are unlikely to be moved by superficial appeals to technological progress. They will not fully accept liquid biopsy until they see substantive demonstrations that the sample-collecting method offers unique benefits to patients, without depriving them of any of the benefits that are already available with traditional biopsy techniques.
Granted, liquid biopsy and traditional biopsy are not mutually exclusive. It is striking, however, that several of the liquid biopsy experts in this roundup editorial said the same thing: Clinicians are looking for assurances that liquid biopsy and traditional biopsy demonstrate concordance. This requirement seems to have the status of a “necessary but not sufficient” condition.
GEN interviewed a number of liquid biopsy experts to learn more about the status of liquid biopsies in the clinic and what they view as some long-term goals for this promising technology.

GEN: What is the biggest obstacle that liquid biopsy must overcome before it wins widespread acceptance as a clinical diagnostic tool?
Ms. Robinson: The need for sufficient medical evidence accrued from prospective clinical studies that can drive a change in the standard of care. Motivating clinicians to order and rely on the technology will require physician education combined with robust clinical evidence that demonstrates the advantages of liquid biopsies for cancer diagnosis, therapeutic decision-making, and monitoring disease progression and a patient’s response to treatment. We need to capture the attention of busy physicians and provide them with the evidence that will convince them to change.
Dr. Patel: One of the biggest challenges is reliably finding tumor DNA in the vast sea of healthy DNA. Clinicians must be convinced that liquid biopsies are as reliable as tissue genotyping and can help patients. The slow uptake of liquid biopsy use in the clinic illustrates the need for more prospective clinical trials that can establish concordance and the real-world utility of liquid biopsy strategies.
The ideal method for liquid biopsy genotyping would both detect and precisely quantify circulating tumor DNA. Unlike next-generation sequencing (NGS), which can primarily identify mutations that are present, Bio-Rad Laboratories’ droplet digital PCR (ddPCR) can provide both qualitative and quantitative information for cancer biomarkers. This is important to clinicians because recent studies at the Dana-Farber Cancer Institute have suggested the amount of mutation present is tied to specific outcomes, including relapse.
Dr. Ionescu-Zanetti: The key obstacle is clinical utility. We need to demonstrate that data obtained from a liquid biopsy provides new, actionable information to the clinician that leads to improved patient outcomes. The first step is demonstrating concordance to primary tumor data to show that the liquid biopsy approach works, and that work is happening now.
We don’t, however, see liquid biopsies replacing traditional biopsies anytime soon. The near-term use will be in situations where liquid biopsies can provide new information. For example, liquid biopsies could be used to identify subclonal mutations or the emergence of resistance mutations. Long term, the technology needs to progress to early-stage patient profiling—that’s where we can really affect outcomes.
Dr. Burke: Pathologists say that “tissue is the issue.” Genomics might point to a BRAF mutation, but in the case of colorectal cancer, BRAF inhibitors don’t work so well—not as well as they do in melanoma. This dependence on tissue is important, but also limiting.
Dr. Manaresi: Evidence of clinical utility will probably drive wider clinical adoption. Although studies have demonstrated that the enumeration of circulating tumor cells (CTCs) is a strong, independent predictor of overall and progression-free survival in metastatic breast, prostate, and colorectal cancer, evidence that treatment decisions based on the enumeration or molecular characterization of CTCs positively impact clinical outcome is still lacking. However, several studies, such as STIC Metabreast and DETECT III, are currently underway and might provide important new insights.
In addition, the lack of standardized methods for genetic analyses of CTCs might also play a role. But this limitation could be overcome by recently developed technologies.
Dr. Lin: With any diagnostic or therapeutic, the key to success is improving patient outcomes. Too often, the temptation is to take a research-use product, one more suited for discovery, and attempt to validate and implement it as a laboratory-developed test, which means trying to find clinical actionability and determine the outcome later. Instead, we need to be precise in our approach to precision medicine. We need to start with the end in mind. Just as there is no one-size-fits-all approach to medicine, there is no one-size-fits-all approach to diagnostics. An understanding of the differences between clinical diagnostic tools and research discovery tools is needed.
Mr. Kazinski: Liquid biopsy will most likely become part of standard diagnostic procedures. Patient monitoring for disease progression, drug-resistance detection, or stratification of patients for a therapeutic regime are likely to be adopted earlier. Applications such as patient screening will probably take a bit longer.
Clinical trial data showing the advantages of liquid biopsy regarding both survival time and quality of life of patients when compared to conventional methods will be one of the major acceptance drivers for adopting liquid biopsy in routine diagnostics. In addition, regulatory approval and reimbursement of tests are essential for the success of this approach.
Ms. Bramlett: The clinical research community is gaining confidence in liquid biopsy results; continuing to build that confidence is critical. Researchers need to know that liquid biopsy results reflect tumor evolution.
The recent availability of Oncomine cell-free DNA assays for next-generation sequencing certainly helps move us in that direction. Making tools like these widely available helps foster the collaboration and sharing of results that can build confidence. And, through rigorous analytical verification of cfDNA analysis methods, the clinical research community is gaining a clear understanding of the advantages and limitations of cfDNA related to cost, false positive results, and a low limit of detection.
GEN: Do you think there is an advantage in the collection method of genetic material for liquid biopsy, that is, cell-free DNA (cfDNA) vs. circulating tumor cells (CTCs)?
Ms. Robinson: In contrast to cfDNA, which is released into the blood when cancer cells die and break apart, CTCs offer a window into the living tumor and the parts that may be resistant to therapy. CTCs can provide information not just on DNA but also on RNA and protein expression and tumor heterogeneity. The problem has been difficulty in harvesting CTCs for analysis. Easy harvest of CTCs is now available using Angle’s Parsortix system. Consequently, there is no longer an advantage in the collection process for cfDNA. Furthermore, both CTCs and cfDNA can now be collected from the same patient sample.
Dr. Patel: Yes. cfDNA is 100 times more abundant in blood than CTCs, but is normally fragmented. Technology advancements to detect cfDNA have been critical in validating this as a cancer biomarker. Small fragments that would have been difficult to examine and detect in the past can now be assessed with high accuracy and sensitivity. The sensitivity of ddPCR for plasma cfDNA is about 70%, and you can detect specific stretches of tumor DNA even at levels as low as 0.1% of total DNA in the blood.
Dr. Ionescu-Zanetti: We don’t believe there is a one-size-fits-all solution. The right sample format will depend on the question being asked, the patient’s cancer type, stage, etc. At Fluxion Biosciences, we have developed solutions for both approaches, and we think they will be complementary. CTCs have the advantage of being enrichable and capable of improving the signal-to-noise ratio, which we find beneficial in early-stage patients where tumor burden is low. cfDNA has the advantage of not requiring a cell isolation step, so the workflow is simpler. But each approach has merits, and we don’t expect to see one dominant approach.
Dr. Burke: CTCs make identification of tissue of origin more tangible—although that’s not to say it’s impossible with cfDNA.
Dr. Manaresi: The information ctDNA and CTCs provide are probably complementary rather than mutually exclusive. ctDNA can detect known mutations in disease-specific genes but does not allow reliable analysis of copy number aberration profiles and cannot detect loss of heterozygosis, which may be important in certain clinical settings. CTCs, on the other hand, may provide important information on the biology of metastasis and allow multi-omic profiling on a cell-by-cell basis and can in this way potentially provide an even more complete picture of tumor biology as compared to analyzing ctDNA alone.
Dr. Lin: Precision diagnostics means tailoring products to specific indications for specific diseases with unmet medical needs. There are some clinical situations where cfDNA is the most appropriate approach, and there are others where CTC is the better fit. CTCs provide a whole cell to analyze but are rare; they are also better suited to explore the functional effects of the cell, such as drug sensitivity and whole-omics profiling. However, cfDNA is often more abundant and better suited for early detection, therapy selection, and resistance and recurrence monitoring. At Natera, we are launching a cfDNA product focused on early detection of recurrence.
Mr. Kazinski: Different analytes have different advantages with respect to the question a diagnostic test is meant to answer. cfDNA can be found and analyzed early and can, for this reason, be more efficient in some cases, such as the detection of residual disease or resistance development. CTCs for most cancers are detectable only at a more advanced stage of disease but allow for genomic, transcriptomic, and phenotypic characterization, providing a more detailed insight into the dynamics and heterogeneity of the disease.
Circulating RNA, either in CTCs or exosomes, can help to correlate genomics and transcriptomic data, allowing for a better understanding of the disease mechanisms.
Ms. Bramlett: Molecular analysis from cell-free nucleic acid tends to be cheaper and faster than a similar analysis from circulating tumor cells. There is increasing use of RNA from cell-free material because together with cfDNA, they enable expanded studies into rare variants, copy number, fusion gene detection, and expression.
Cell-free nucleic acid has many of the same advantages as CTCs; the primary difference is whether protein markers or location information is important in the analysis.
This suggests that there are situations where both cfDNA and CTC sources can be beneficial. For example, are copy number variations present in the same cell as a specific single nucleotide variant? Are cells displaying PDL-1 AB binding sites also harboring driver mutations? Answers to these questions could change therapy decisions in the future.
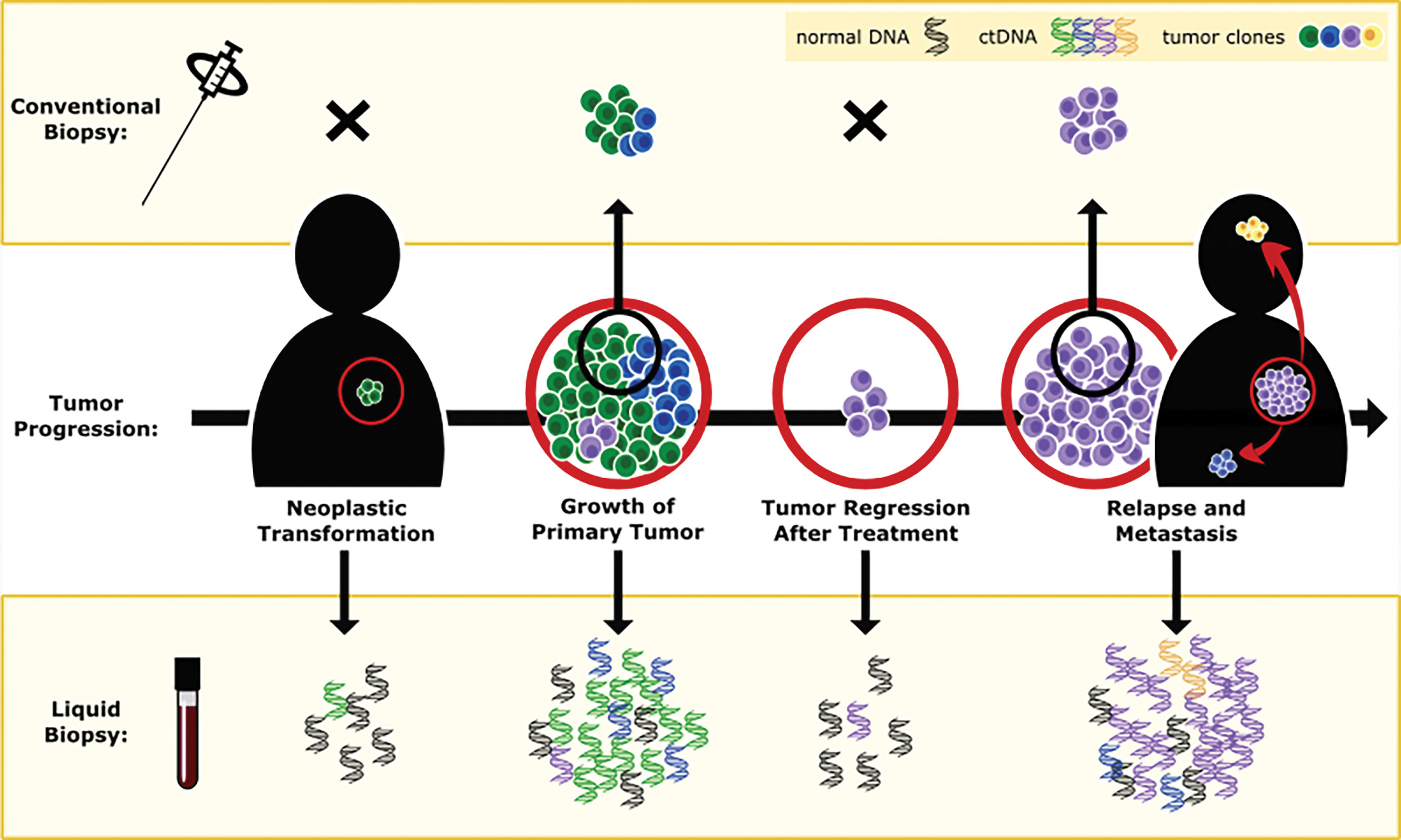
GEN: Should the goal of all liquid biopsies be to eventually become completely noninvasive? Are there substantial reasons to stick with blood as the medium of choice to isolate genetic material?
Ms. Robinson: Compared to acquiring a tumor biopsy sample, the current standard of care for cancer diagnosis, obtaining a blood sample for liquid biopsy, is relatively noninvasive. For the majority of cancers, it is hard to imagine another bodily fluid that is easier to collect and could replace blood for the purposes of liquid biopsy—and that could be used to collect intact tumor cells for downstream molecular analysis.
Dr. Patel: It would depend on the cancer. For some cancers, completely noninvasive testing such as urine sampling is possible. But blood-based testing is an integral diagnostic modality for cancers such as lung and breast cancer.
A blood draw is generally considered noninvasive or minimally invasive, and will most likely remain a major medium for isolating genetic material for the foreseeable future.
Dr. Ionescu-Zanetti: We haven’t found that obtaining blood samples is too invasive or challenging in most current settings, where the patient is already in the clinic and obtaining blood is straightforward. A totally noninvasive sample format, urine for example, for early screening or frequent sampling post-surgery, could lead to an interesting consumer-driven self-sampling model, but we don’t see that happening any time soon.
Dr. Burke: There are several mediums from which cells and DNA can be identified including urine. It really comes down to what is in the best interests of the patient—noninvasive and accurate testing is the way to go.
Dr. Manaresi: While noninvasively obtainable body fluids, such as urine or sputum, hold the promise of complete noninvasive sampling, these are probably only suitable for tumors in nearby tissues. Blood as source of tumor material has the potential to be applicable to a wide range of tumor types. Blood-based liquid biopsy can provide complementary information to traditional diagnostic tools in multiple settings, and when the pathological tissue is inaccessible, it could be the only source of tumor information.
Dr. Lin: What ultimately matters is patient outcomes. If an invasive method improves survival more than a noninvasive approach, the collection method becomes less important. Similarly, if a noninvasive method performs better, whether due to ease of collection or other unrelated factors, it should be adopted. We should all approach improvements with patient outcomes in mind.
Mr. Kazinski: For certain specific diseases, such as kidney or bladder cancers, urine may serve as a sufficient source of relevant markers in the future. However, blood will most likely be the primary source for isolating and analyzing circulating biomarkers. The main reason is that blood allows not only the analysis of biomarkers of interest, but also the measurement of other clinically relevant parameters such as coinciding inflammation.
Ms. Bramlett: There are currently several technologies that reduce risks while helping to build knowledge that leads to evaluation of the clinical utility of liquid biopsies. Historically, we have obtained that information from solid tumor samples. Today, we talk about blood as a primary source of genetic material, but it’s easy to think about other bodily fluids providing the same source material.
These other sources raise additional questions beyond risk and invasiveness. The biology of a tumor is important—it affects the amount and quality of available source material. As such, we need to continue to develop flexible technologies that enable clinical researchers to investigate these options more fully.




