Scientists in the U.S. and Portugal have developed a method for growing adult-like human heart muscle from blood-derived human induced pluripotent stem cell–derived cardiomyocytes (hiPS-CMs), in just four weeks. The resulting heart muscle displayed features, including adult-like gene expression and an organized ultrastructure, which haven’t been demonstrated using other methods for growing human heart tissue in the laboratory.
Key to the Columbia University–led team’s achievement was the use of early-stage hiPS-CMs that have only just started to contract spontaneously, and subjecting these cells to an increasingly intense conditioning regime that triggers the developing heart tissue to mature. “The common approach in our field has been that the more mature the starting cardiomyocytes, the better,” says Kacey Ronaldson-Bouchard, Ph.D., first author of the researchers’ published paper in Nature. “However, we found that very early-stage cells, which still have developmental plasticity, would respond better to the external signals we deliver to drive maturation.” Dr. Ronaldson-Bouchard is a postdoctoral scientist in the laboratory of senior investigator Gordana Vunjak-Novakovic, Ph.D., the Mikati Foundation Professor at Columbia Engineering, and professor of medicine at Columbia University Vagelos College of Physicians and Surgeons.
The researchers suggest that the adult-like tissue generated using their method could represent a major new tool for basic research, disease- and drug-related studies. They report their achievement in a paper entitled, “Advanced maturation of human cardiac tissue grown from pluripotent stem cells.”
None of the current methods for culturing human heart muscle is capable of generating tissue that recapitulates the physiology of the adult myocardium, the researchers note. And while hiPS-CMs can be matured by long-term culture and using electrical, hydrodynamic, and mechanical stimulation, studies suggest that this process of in vitro maturation doesn’t follow how heart tissue develops in vivo. With this in mind, the Columbia University team, working with scientists at the University of Minho in Portugal, set out to investigate why current strategies fail, by comparing the in vitro maturation of human cardiac tissues derived from early-stage hiPS-CMs that have just started to contract, with strategies that use much later-stage hiPS-CMs that have been matured in culture.
They encapsulated the early- and later-stage hiPS-CMs in a fibrin hydrogel stretched between two flexible pillars, and supported the setup in a multi-chamber organ-on-a-chip platform. They then subjected the cells to different regimens of electrical pacing and mechanical force—generated by pulling the elastic pillars—to mimic conditions in the developing heart. The applied electrical stimulation effectively forced the heart muscle to twitch and pull against the pillars. Three conditioning regimens were applied. Under control conditions, the cells were not subjected to any electrical stimulation. Another regimen involved stimulation at a constant frequency for three weeks, while tissue developing under the third, “intensity training” regimen was subjected to increasing strength of stimulation over the three week training period. This was designed to mirror the mechanical loading that the heart muscle would experience during the fetal to postnatal transition.
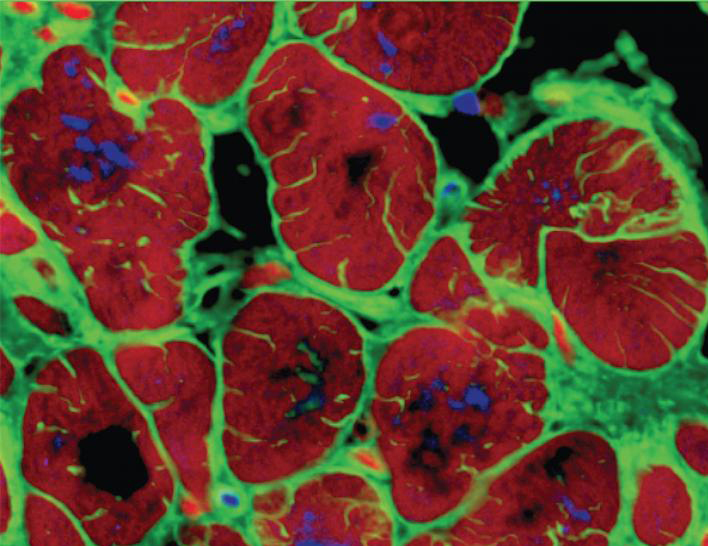
The results showed that intensity-trained tissues grown from early-stage hiPS-CMs matured quickly to display “remarkably organized ultrastructure,” and structural features characteristic of adult heart tissue, the team states. Such features included the physiological length of the sarcomeres, density of mitochondria, the presence of transverse tubules, and the switch to oxidative metabolism. “The increasing contractile demands induced the adult-like cardiac morphology that is necessary for high force generation in early-stage intensity-trained tissues,” the team writes. “The cell size increased (an indicator of physiological hypertrophy) and both cells and nuclei were elongated (an indicator of maturation). The sarcomere length reached 2.2 μm, a similar value to that of adult human ventricular myocytes.”
The intensity-trained early-stage hiPS-derived cardiac tissue also displayed changes in the expression of genes associated with adult-like conduction, maturation, ultrastructure, energetics and calcium handling, the authors state. “The contractile capacity, fraction of cells containing sarcomeres and organization of sarcomeric α-actinin also resembled those of adult human myocardium…Early-stage intensity-trained tissues also exhibited a positive force–frequency relationship (FFR), a hallmark of maturation not seen in other in vitro myocardial tissue models.”
In contrast, all the other early-stage derived tissues, and all the late-stage-derived tissues exhibited immature cardiac phenotypes.
As well as allowing environmental and molecular control and the application of defined physical stresses on the developing tissue, the team’s platform also means that optical measurements of functional properties, frequency, amplitude, and force of contractions can be taken, along with measurements of calcium signal propagation.
“Many of the ongoing efforts, including those from our lab, have been biomimetic in nature, trying to recapitulate the known events present during native development,” Dr. Vunjak-Novakovic states. “Because these efforts have been limited in how much maturation can be achieved, we decided to try something totally new: to explore the concept of accelerated development. It took a lot of creative thinking and clever engineering by the whole team across both campuses of Columbia University to develop the model we now have: a highly matured, patient-specific heart muscle that can be used for studies of heart development, physiology, disease, and responses to drugs.”
Dr. Vunjak-Novakovic’s team has demonstrated use of the resulting human heart tissue to recapitulate the phenotype of specific heart conditions, including pathological hypertrophy or reduced cardiac contractility associated with calcium-channel mutations. The researchers are expanding their work on disease modelling, into areas such as cardiotoxicity induced by non-cardiac drugs. The hope is that their work could help to identify new therapeutic targets and enable the development of drugs that can cure heart disorders or protect the heart against drug toxicity.
The research is part of the “organs on a chip” project funded by the National Institutes of Health (NIH). “The resulting engineered tissue is truly unprecedented in its similarity to functioning human tissue,” comments Seila Selimovic, director of the National Institute of Biomedical Imaging and Bioengineering (NIBIB) Tissue Chips program, within the NIH that funded this research. “The ability to develop mature cardiac tissue in such a short time is an important step in moving us closer to having reliable human tissue models for drug testing.”