By Subhanjan Mondal, PhD

Senior Research Scientist
Promega
Wastewater-based epidemiology (WBE) is the analysis of wastewater to identify the presence of biologicals or chemicals for the purpose of monitoring public health. WBE has previously been used for detecting pharmaceutical and industrial waste, as well as monitoring rates of opioid abuse within communities.1 Several countries have even successfully limited the spread of polio and hepatitis A outbreaks using WBE.2,3
During the COVID-19 pandemic, WBE emerged as a critical epidemiological tool for gathering population-wide data on infection rates. Researchers found early in the pandemic that SARS-CoV-2 genetic material could be detected both in the feces of infected individuals and, later, in wastewater samples.4,5 However, no culturable SARS-CoV-2 virus was found in the wastewater. This led many research groups and public health organizations to investigate wastewater surveillance systems for monitoring the SARS-CoV-2 levels in sewage systems.
This type of testing offers several unique advantages compared with patient clinical testing. First and foremost, it functions as an early warning system. A recent study found that SARS-CoV-2 RNA concentrations in wastewater rose as much as eight days earlier than clinical test results.5 The data also provides a snapshot of an entire community, instead of relying on individuals who choose to be tested. When sampled continuously, wastewater provides a picture of the overall infection trends of the population, allowing local officials to determine whether current public health guidelines are meeting the needs of the moment.
All of these factors led to an increase in demand for efficient, reliable methods for detecting SARS-CoV-2 in wastewater.
Challenges of WBE
Unfortunately, extracting high-quality nucleic acids from wastewater can be uniquely difficult. The concentration of SARS-CoV-2 genetic material in a sample is extremely low, so sample concentration is often a prerequisite for detection and quantitation. Many of the traditional methods for concentrating viruses, such as PEG/NaCl precipitation and centrifugal ultrafiltration, may require inconveniently large sample sizes or result in inconsistent rates of viral recovery and co-purification of PCR inhibitors. With concentrations already extremely low, it is critical to extract as much high-quality RNA as possible to generate a reliable signal.
In addition, wastewater contains many materials that can easily interfere with any step in the wastewater testing process. Detergents, chaotropic agents, bile salts, and urea may cause structural changes to proteins and nucleic acids. The altered molecules may associate with suspended solids such as the cellulosic component of the diet and tissue paper. However, the fact that we were able to detect SARS-CoV-2 genetic material in wastewater indicates that most of the nucleic acids are present in ribonucleoprotein complexes and thus protected from degradation.
Finally, an effective wastewater surveillance system should meet the specific needs of the laboratory staff who will be performing the testing. This includes optimizing sample volumes, simplifying the workflow, minimizing specialized equipment needs, and expediting the time from sample to results. The workflow should also be scalable to meet any throughput needs.
Direct capture of SARS-CoV-2 RNA
To address some of these challenges, our team developed a new system for extracting RNA from wastewater.
We focused on direct capture of total nucleic acid (TNA), which we believed would be unbiased toward all available nucleic acids. Chaotropic agents are added to raw sewage, allowing binding onto a silica matrix. The captured nucleic acids are subjected to successive alcohol washes to remove RT-qPCR inhibitors and eluted in water. The eluted sample can then be further processed using manual or automated nucleic acid purification chemistry.
To better capture nucleic acids associated with solids in wastewater samples, we added a proteolytic cleavage step to the beginning of the workflow. This resulted in a twofold increase in the number of SARS-CoV-2 genome copies extracted from the samples.
To test the new system, wastewater samples were processed using our direct capture method and PEG/NaCl precipitation. We observed a 20-fold increase in the yield of SARS-CoV-2 RNA using our direct capture methods. This was particularly noteworthy because now we could get larger nucleic acid yields from smaller sample volumes.
Real-world testing
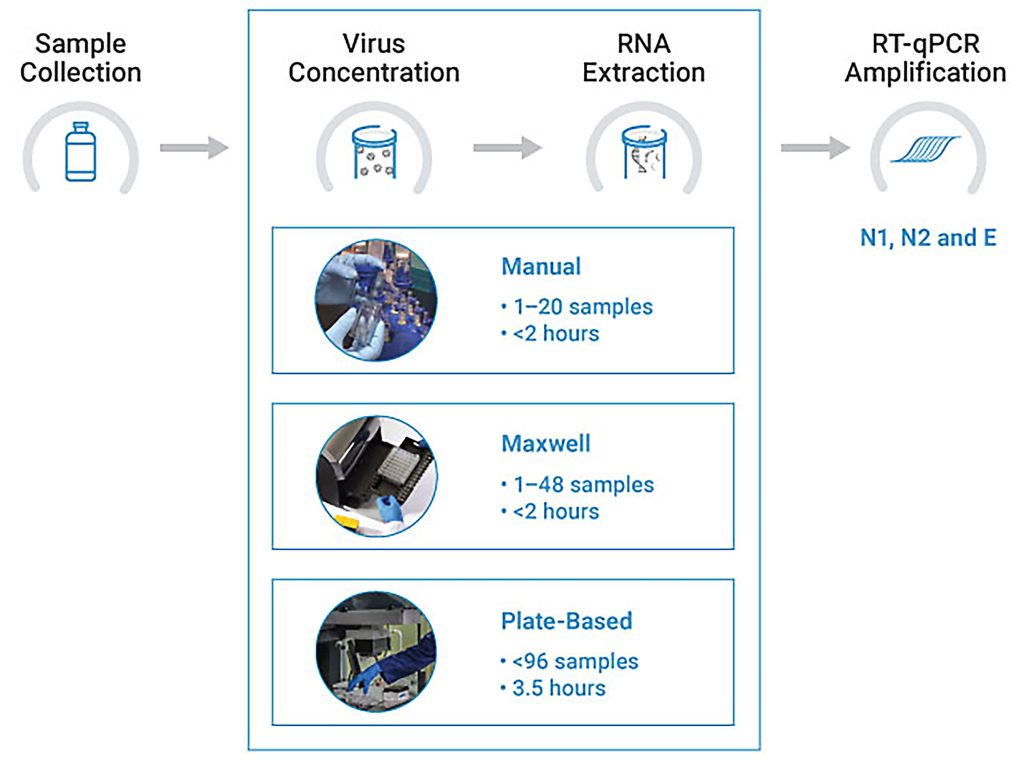
We applied the direct capture methods in three wastewater treatment plants in Dane County, WI, from October 2020 to January 2021. This time period included a significant wave of infections in the county, with clinical testing indicating as many as 500 new cases per day. Samples were processed using the methods described above, and we analyzed SARS-CoV-2 RNA levels via RT-qPCR using N1, N2, and E targets.
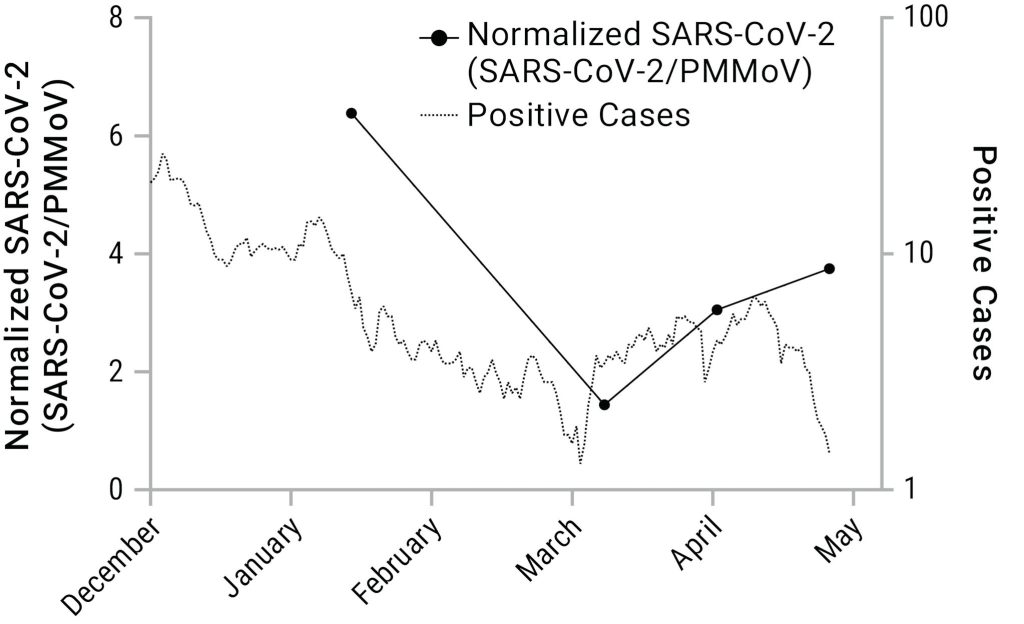
Our results over the sampling period showed high correlation with the trends in COVID-19 cases reported by the municipality. The peak of the SARS-CoV-2 genetic signal in wastewater is concurrent with the peak of positive SARS-CoV-2 clinical tests reported in mid-November of 2020. The results demonstrate the potential for wastewater-based surveillance of community spread using our direct capture methods and RT-qPCR-based detection.
Finally, we determined that our direct capture purification method yielded nucleic acids that were compatible with next-generation sequencing for variants of concern. Using a commercially available SARS-CoV-2 amplicon panel and associated library preparation kit, we were able to detect variants that were consistent with known variants in Dane County during the period of sampling.
These methods and results were described in November 2021 in Science of the Total Environment.6 We have also commercialized these methods in several kits offered by Promega—the Wizard® Enviro TNA Kit for manual purification, the Maxwell® RSC Enviro TNA Kit for Maxwell® automation with 1–48 samples, and the Maxwell® HT Environmental TNA Kit for high-throughput, plate-based automation. We have also developed and launched GoTaq® Enviro Wastewater SARS-CoV-2 kits for detection of either N1, N2, or E by RT-qPCR, which include standards and appropriate controls for wastewater testing. These purification and amplification kits together make up a complete workflow for detecting SARS-CoV-2 in wastewater samples.
Subhanjan Mondal, PhD ([email protected]), is a senior research scientist at Promega.
References
- Lorenzo M, Picó Y. Wastewater-based epidemiology: current status and future prospects. Curr. Opin. Environ. Sci. Health 2019; 9: 77–84. DOI: 10.1016/j.coesh.2019.05.007.
- Hovi T, Shulman LM, van der Avoort, H, et al. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol. Infect. 2012;140(1): 1–13. DOI: 10.1017/S095026881000316X.
- Hellmér M, Paxéus N, Magnius L, et al. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014; 80(21): 6771–6781. DOI: 10.1128/AEM.01981-14.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med. Virol. 2020; 92(7): 833–840. DOI: 10.1002/jmv.25825.
- Peccia J, Zulli A, Brackney DE, et al. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020; 38(10): 1164-1167. DOI: 10.1038/s41587-020-0684-z.
- Mondal S, Feirer N, Brockman M, et al. A direct capture method for purification and detection of viral nucleic acid enables epidemiological surveillance of SARS-CoV-2. Sci. Total Environ. 2021; 795: 148834. DOI: 10.1016/j.scitotenv.2021.148834.