We’re already familiar with the three blind mice, but we never hear about blind zebrafish. Perhaps that’s because zebrafish have admirably flexible retinal stem cells called Müller glia (MG). In zebrafish, these cells can replenish damaged retinal neurons and restore vision. In mice and other mammals, however, Müller glia usually refuse to differentiate into retinal neurons. Sometimes they do, to a limited extent, in response to injury, which raises interesting questions: Could Müller glia be activated by less drastic means, and could these cells even serve, potentially, as a way to reverse blinding diseases? To answer these questions, scientists based at the Icahn School of Medicine at Mount Sinai tried using genetic factors to stimulate the regenerative machinery in Müller glia.
The scientists succeeded in changing Müller glia into rod photoreceptors. The scientists also reversed congenital blindness in mice.
Detailed findings appeared August 15 in the journal Nature, in an article titled, “Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas.” This article describes a two-stage reprogramming process. First, Müller glia in normal mice were induced to divide by injecting the eyes of the mice with a gene to turn on a protein called beta-catenin. Weeks later, the mice's eyes were injected with factors that encouraged the newly divided cells to develop into rod photoreceptors.
“We report that following gene transfer of β-catenin, cell-cycle-reactivated MG can be reprogrammed to generate rod photoreceptors by subsequent gene transfer of transcription factors essential for rod cell fate specification and determination,” the article’s authors wrote. “MG-derived rods restored visual responses in Gnat1rd17Gnat2cpfl3 double mutant mice, a model of congenital blindness throughout the visual pathway from the retina to the primary visual cortex.”
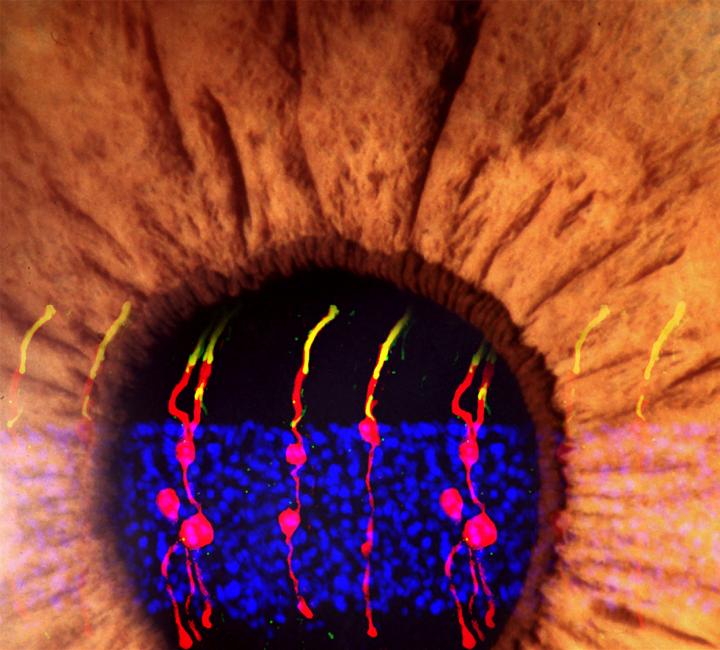
The researchers, led by Bo Chen, Ph.D., associate professor of ophthalmology and director of the Ocular Stem Cell Program at the Icahn School of Medicine, used microscopy to visually track the newly formed cells. They found that the newly formed rod photoreceptors looked structurally no different from real photoreceptors. In addition, synaptic structures that allow the rods to communicate with other types of neurons within the retina had also formed. To determine whether the Müller glia-derived rod photoreceptors were functional, they tested the treatment in mice with congenital blindness, which meant that they were born without functional rod photoreceptors.
In the treated mice that were born blind, Müller glia-derived rods developed just as effectively as they had in normal mice. Functionally, they confirmed that the newly formed rods were communicating with other types of retinal neurons across synapses. Furthermore, light responses recorded from retinal ganglion cells—neurons that carry signals from photoreceptors to the brain—and measurements of brain activity confirmed that the newly-formed rods were, in fact, integrating into the visual pathway circuitry, from the retina to the primary visual cortex in the brain.
“From a practical standpoint, if you're trying to regenerate the retina to restore a person's vision, it is counterproductive to injure it first to activate the Müller glia,” says Dr. Chen. “We wanted to see if we could program Müller glia to become rod photoreceptors in a living mouse without having to injure its retina.”
Dr. Chen's lab is conducting behavioral studies to determine whether the mice have regained the ability to perform visual tasks such as a water maze task. Dr. Chen also plans to see if the technique works on cultured human retinal tissue.