A treatment for Duchenne muscular dystrophy (DMD), carried out through genetic repair, may be on the horizon, according to researchers in a new paper published in Molecular Therapy—Methods & Clinical Development (“A Five-Repeat Micro-Dystrophin Gene Ameliorated Dystrophic Phenotype in the Severe DBA/2J-mdx Model of Duchenne Muscular Dystrophy”).
The treatment approach seeks to correct the faulty genes that lead to a deficiency of dystrophin, a protein that is necessary for muscle fiber strength.
The researchers say their approach to treat DMD is novel, specifically because the gene transfer they describe—meant to replace damaged code with a functional version of dystrophin—uses an adeno-associated virus (AAV) vector to shuttle the replacement genes into cells.
“This is the first AAV vector that utilized a novel muscle-specific promoter to drive a codon-optimized microdystrophin cDNA that carries the critical neuronal nitric oxide synthase (nNOS)-binding domain to prevent ischemia and fatigue during muscle contraction,” study leader Dongsheng Duan, Ph.D., University of Missouri and Solid Biosciences, tells GEN. “There have been other gene-transfer vectors attempted in the past (such as adenoviral vector, herpes simplex virus, and plasmid), but they have largely been unsuccessful due to the complexity of the disease, challenges associated with delivery, and the large size of the native dystrophin gene….While AAV has also been used in the past, none of the previous AAV vectors have the combinatoral features mentioned above.”
The researchers say AAV is a feasible gene-delivery vehicle because of its ability to reach muscle tissue in DBA/2J-mdx mice (a mouse model that is characterized by symptoms similar to DMD in humans). But, because AAV is fairly small in size, it is difficult to pack all of the necessary genetic material into the vector. In earlier models, researchers used miniature versions of the dystrophin gene in AAV, but there were limitations to this approach, and missing genetic material in that mouse model precluded its practical use. New models, spearheaded by Dr. Duan, include the previously missing nNOS-binding domain, allowing the proper supply of blood to flow to muscles when they contract. The microgenes that fit into the AAV vector are one-third the size of regular dystrophin.
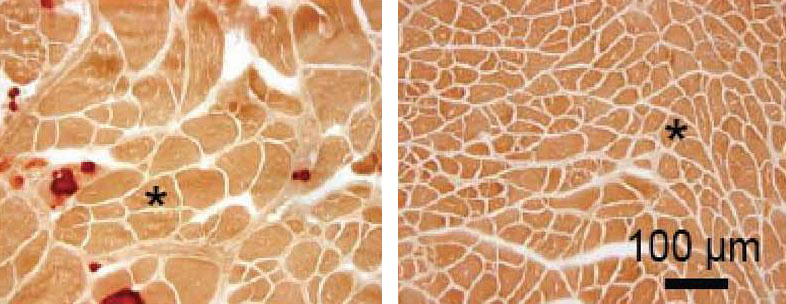
Fifteen weeks following injection with the micro-dystrophin-laden AAV vector, all 10 of the mice that were treated had levels of microdystrophin protein in their skeletal muscles, and some of the symptoms associated with DMD were significantly reduced. Treatment with micro-dystrophin and its effects on the mice’s hearts, however, could not accurately be measured, because of issues with heart calcification in the control mice.
“For the vast majority of DMD patients, there are no satisfactory symptomatic or disease-modifying treatments,” Dr. Duan says. He notes that glucocorticoid treatment is the current standard of care; while these treatments have been able to temporarily improve muscle strength, prolong ambulation, and slow the progression of DMD, they do not address the underlying genetic cause of DMD. In addition, notes Dr. Duan, glucocorticoid use is associated with well-known adverse events, such as severe weight gain, stunted growth, weakening of bone structure, and metabolic dysfunctions, among others.
Some believe that to correct a genetic defect permanently, a gene therapy has to be stem-cell based. But Dr. Duan says that while correction of the gene mutation in muscle stem cells would allow regenerated muscle cells to carry a corrected gene and express a functional dystrophin protein, it is not the only option for a lifetime fix. “Human studies have shown that one-time intramuscular injection of an AAV vector can result in the expression of a therapeutic protein for many years (for example, a study showed Factor IX expression for 10 years in a hemophilia patient). In preclinical studies in murine and canine models, we have also observed persistent multiyear microdystrophin expression from AAV vectors. In the case of mice, a single injection can lead to microdystrophin expression throughout the lifespan.”
There is concern, of course, that viruses will insert their genetic payloads incorrectly, spurring some dramatic, and perhaps life-threatening, reactions. “So far, there is no concrete evidence suggesting AAV genome integration in muscle cell,” Dr. Duan explains. “Both wild-type and recombinant AAV have been shown to exist as episomal molecules in muscle. Insertional mutagenesis is minimal for an AAV vector. This is in sharp contrast to retrovirus-based vectors, which require integration of the genome in the host genome to express therapeutic proteins.”