Co-Founder and CEO
Antibody-drug conjugates (ADCs) have been in development for decades, but only about a dozen have received FDA approval. Clearly, ADCs still have some major challenges. Yet they have done little to deter developers. Indeed, approximately 50 biopharma companies are developing or partnering to develop ADC drugs. One of these companies is Araris Biotech. It offers a novel ADC linker technology for conjugating cytotoxic payloads and native antibodies. According to the company, the technology obviates time-consuming antibody engineering projects.
“ADCs look simple on paper,” says Philipp Spycher, PhD, co-founder and CEO of Araris Biotech. “But they have turned out to be complicated.”
ADC developers struggle to find the right combinations of antibody, linker, and payload. In addition, ADC developers contend with solubility problems. (ADCs tend to cluster together in solution.) Finally, ADC developers must cope with various linkage issues. For example, ADCs with unstable linkages tend to lose their payloads, reducing drug efficacy and increasing toxicity. Also, linkages can alter the properties of parent antibodies, contributing to off-target effects.
It is by addressing linkage issues that Araris Biotech intends to distinguish itself from its competitors. “We have some key advantages that separate us from anybody else,” Spycher asserts. “Our proprietary technology allows the ADC to behave like a normal antibody, as if the payload isn’t attached, so it can deliver high amounts of toxins to the tumors.”
A de novo approach
Araris Biotech’s technology enables the antibody to protect the attached payload, shielding it from protein interactions until it reaches the target tissue where the payload is released. Preclinical studies show a four- to sixfold improvement in the therapeutic index compared to commercially available ADCs.
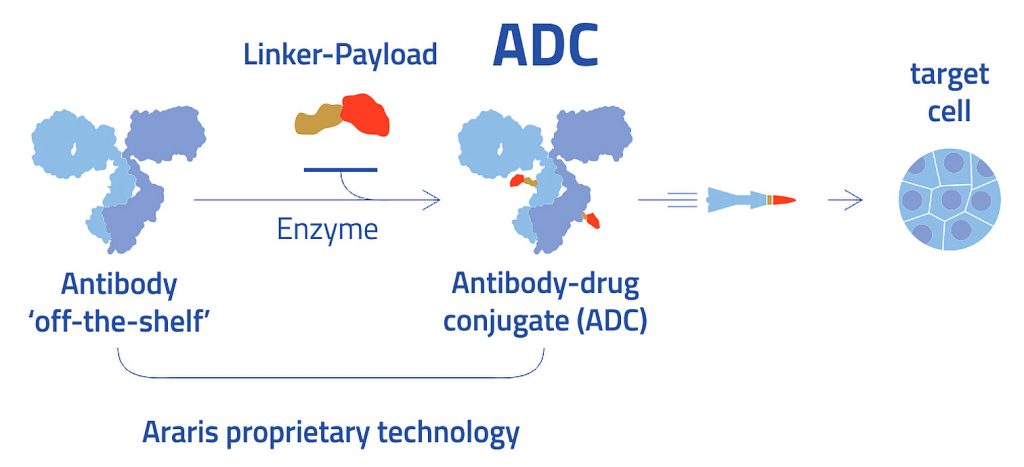
The genesis of this approach was in Spycher’s postdoctoral research, which showed that transglutaminase could be used for the antibody conjugation. Historically, many research groups and companies used transglutaminase with nonpeptidic linkers, where there was a need to engineer or modify the antibody for successful conjugation. Today, transglutaminase may be used to accomplish antibody conjugation with peptide linkers. Indeed, the linkers can affix drug payloads directly to “off the shelf” antibodies. An ADC can be assembled in one step, without the need to engineer the antibody to accept the payload.
High-drug-load conjugation is another option, Spycher points out. Here, the linker can be used to double the payload attachment sites from one to two or even higher, or it can enable the attachment of two different payloads without increasing the number of attachment sites on the antibody.
“The beauty of our technology is that it enables the use of off-the-shelf antibodies that link to any payload,” Spycher maintains. Payloads can be linked to antibodies within one day. “Everybody said it wouldn’t be possible,” he relates. In this case, everybody was wrong.
“Last person” in the field
Spycher developed the basis of this ADC approach as a postdoctoral fellow at the Paul Scherrer Institute (PSI) in Switzerland. He says that his professor had an “everything possible has already been invented” moment and waxed pessimistic about peptide linker research. “He said that I would be the last person working on peptide linkers,” Spycher recalls. “He didn’t believe anything more could come from the field.”
The professor took Spycher on with the understanding that the young postdoctoral fellow would spend 80% of his time on the professor’s priorities and only 20% of the time on peptide linkers. Nonetheless, Spycher found that by using peptide linkers, there was no need to engineer the antibody beforehand. This discovery, which came as a “complete surprise,” shifted priorities within the laboratory. Spycher was allowed to devote more time to his own ADC work.
Three years ago, Spycher’s peptide linker work spun out from PSI and ETH Zurich. The main reason for that move was Spycher’s great desire to incorporate a company.
The decision wasn’t made lightly. Spycher had received a grant as a postdoctoral fellow that put him in touch with pharmaceutical companies and thus enabled him to assess the market potential of his ADC technology. “I saw interest from both pharma companies and investors,” Spycher notes. “Then the idea got traction from highly experienced people who wanted to join us. That was very helpful.”
Araris shows its mettle
For any small, young company, establishing credibility and enhancing visibility are constant challenges. Araris Biotech is beginning to make its mark. Last autumn, it was shortlisted for the 8th World ADC Awards, ranked number 11 in the top 100 Swiss startups, and it listed among the top 15 Swiss biotech startups making a difference in Europe by Labiotech.eu.
Perhaps the most important distinction, however, was the company’s ability to continually generate data. Data lends credibility, Spycher stresses. “And it’s better when it comes from a collaboration,” he continues. “Once that occurred, we gained significant interest from investors.” A $16 million seed round that included Swiss and U.K. investors was closed in 2020. This fundraising deal stands out, he says, because it enabled the company to generate even more data, and to strengthen its management team and board of directors.
The most recent additions to the Araris Biotech board are Jeff Sharman, MD, who pioneered the use of B-cell receptor antagonists, and Clive Stanway, PhD, an independent advisor with expertise in discovery and development and commercialization of oncology therapeutics.
Now, with an established management team, accomplished board members, and generous financing, Araris Biotech is garnering interest from multiple pharmaceutical companies. “They are happy, so we are happy,” Spycher reports. “We hope to soon move to the next stage.” Doing so entails transforming the interest from pharma companies into out-licensing agreements.
Scientifically, Araris Biotech is enhancing the stability and targeting of its existing linker technology as well as developing additional linker approaches. Moreover, the company is working to nominate a lead molecule and is looking at potential targets among liquid tumors. Spycher says that he expects toxicology and tolerability studies in nonhuman primates to begin this spring.
The company’s peptide linker technology has the potential to notably ease the challenges associated with ADCs. Specifically, the technology could enable Araris and its partners to use off-the-shelf antibodies while achieving tightly targeted delivery, enhanced effectiveness, and limited side effects. “And that,” Spycher declares, “is the holy grail for better therapeutic indices.”