The practice of bottom-up proteomics relies to a large extent on the separation performance that can be achieved with state-of-the-art nano LC-MS/MS equipment. Depending on the sample complexity or the instrument time that can be dedicated to a certain sample, different LC columns and corresponding LC-MS/MS methods are often required. When comprehensive proteome analysis with deep coverage is needed, relatively long columns (lengths up to 75 cm) are typically operated with long and shallow solvent gradients, delivering the highest chromatographic performance.
This is indeed a good strategy if very complex samples need to be analyzed and when as much information as possible needs to be retrieved from these samples. However, routine daily proteome analysis often deals with simpler samples or demands increased sample throughput, making total analysis times above 120 min undesirable or even impossible.
As an alternative to the conventional packed-bed nano LC column, PharmaFluidics offers a micromachined nano LC chip column called the micropillar array column (µPAC™). The inherent high permeability and low on-column dispersion obtained by the perfect order of the separation bed is what sets µPAC-based chromatography apart from conventional chromatography. The peak dispersion originating from heterogeneous flow paths in the separation bed is eliminated (no A-term contributions), and therefore, components remain much more concentrated during separation. The freestanding nature of the pillars also leads to much lower backpressure, allowing the use of very long columns with exceptional peak capacities.
To complement its innovative 200-cm-long column, which is designed to perform comprehensive and sometimes time-consuming proteome research, PharmaFluidics has introduced a 50-cm-long µPAC column. It can be used in more routine research. With an internal volume of 3 µL, this column is suited to perform high-throughput analyses with shorter gradient solvent times (30-, 60-, and 90-minute gradients), and it can be used over a wide range of flow rates, between 100 and 2000 nL/min.
Materials and Methods
The separation performance of two state-of-the-art packed-bed nano LC columns (150 × 0.075 mm, sub-2-μm porous silica particles obtained from two different vendors) and a 50-cm-long μPAC column were evaluated by analyzing 500 ng of a tryptic digest originating from a human cell lysate. For every column, separation was performed using three different gradient times (30, 60, and 90 min) in triplicate.

The Pierce™ HeLa Protein Digest Standard (P/N 88328) from Thermo Fisher Scientific was obtained in lyophilized form. Twenty micrograms of lyophilized peptide material was reconstituted in 40 µL of 0.1% formic acid in LC-MS-grade water to a concentration of 500 ng/µL. Samples were spiked with Pierce™ Retention Time Calibration Mixture (0.5 pmol/µL; P/N 88320) to a final concentration of 50 fmol/µL. Freshly prepared protein digest standard was used for each column type.
All columns were positioned in the column compartment of a Thermo Fisher Scientific UltiMate™ 3000 RSLCnano system and maintained at 50 °C during the entire experiment. The nano LC system was configured to perform direct injection of a 1-µL sample onto the column. All columns were operated at a flow rate of 300 nL/min. In addition, the 50-cm µPAC column was operated at a flow rate of 1000 nL/min, as much lower flow resistance is observed for this type of column. A nonlinear gradient from 1 to 50% of solvent B (0.1% formic acid in 80% LC-MS-grade acetonitrile) was applied over the allotted gradient times of 30, 60, and 90 min.
For these experiments, the nano LC system was coupled to a Thermo Fisher Scientific Orbitrap Elite™ Hybrid Ion Trap-Orbitrap mass spectrometer by using a nanoFlex ion source. All columns were connected to a New Objective PicoTip™ emitter, and for the 50-cm µPAC column, a grounded connection was provided between the outlet union and the mass spectrometer. For all columns, the voltage required for electrospray ionization was applied on a 50-µm through-bore stainless-steel union through a liquid junction.
Results
One of the main goals of this experiment was to benchmark the separation performance of the 50-cm µPAC column against two commonly used packed-bed columns obtained from different vendors. Rather than aiming for extremely deep proteome coverage, the 50-cm µPAC column is designed for proteome research where improvements in reliability and throughput are needed in addition to excellent chromatographic performance.
Elution profiles for tryptic digest sample.
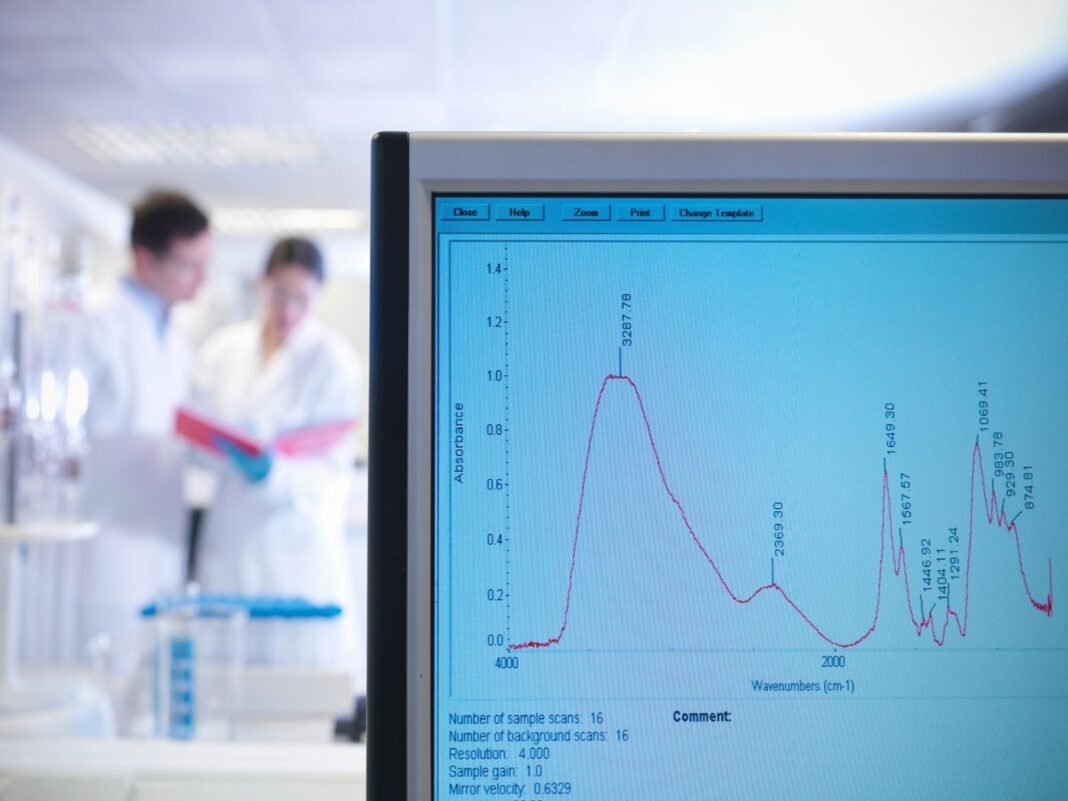
MS base peak chromatograms obtained for 60-min gradient separations (total run time: 90 min) of 500-ng HeLa protein digest standard are shown in Figure 1. Even though very similar relative abundances were found for the conventional packed-bed columns (1.5 × 108), the highest value (2.5 × 108) was from the 50-cm µPAC operated at a 300-nL/min flow rate. The 50-cm µPAC column operated at 1000 nL/min exhibited a slightly lower overall relative abundance (1.0 × 108), but the increased flow rate resulted in a substantial reduction of the column void time.
Because of the low column backpressure inherent to µPAC column backbones, chromatographic separations on 50-cm µPAC columns can be performed at elevated flow rates, that is, flow rates of up to 2000 nL/min. This maximum column backpressure of the µPAC column (even when operated at 1000 nL/min) is exceptionally low compared to the backpressures observed for the packed-bed columns. With stated backpressures around 40 bar, this represents more than a sixfold reduction of the column backpressure for the 50-cm µPAC column.
Chromatographic performance observed for peptide retention time standards.
Besides high flow rate flexibility (100–2000 nL/min) and low column backpressure, excellent peak shapes were found with the 50-cm µPAC column. Based on the 15 reference peptides from the Pierce™ Retention Time Calibration Mixture, average peak widths ranging from 0.13 to 0.22 min (measured at 13.5% height or 4σ) were observed, which is remarkably low compared to both packed-bed column types. This difference is even more pronounced when the µPAC column is run at a flow rate of 1000 nL/min.
Calculation of the peak capacity revealed higher capacities for the µPAC column compared to both conventional nano LC columns for all gradient durations tested. The exceptionally high peak capacity value of 226 obtained for a 30-min gradient separation at a flow rate of 1000 nL/min highlights the improvement of this 50-cm µPAC column over the traditional packed-bed columns frequently used in routine daily proteomics research.
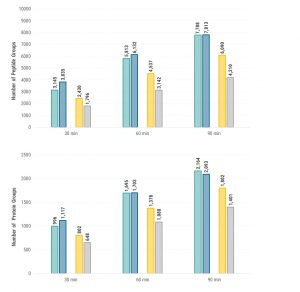
Increased proteome coverage.
Acquired MS/MS spectra were screened against the human reference database (UniProt) by using the Thermo Fisher Scientific Proteome Discoverer™ 2.2 platform with a false discovery rate of 0.1%. More than 1700 protein groups (based on 6100 peptide groups) could be identified from the 50-cm µPAC column when a 60-min gradient separation profile was used (Figure 2). Compared to the two conventional packed-bed columns, this is an average increase of 40% in protein and 60% in peptide identifications. In line with the improved chromatographic performance (reduced peak width and greater peak capacity) observed with the µPAC column, higher proteome coverage, both at the protein and the peptide level, was observed for all conditions tested in this experiment.
Conclusions
Compared with conventional packed-bed column technology, µPAC technology offers several advantages in robustness, operational flexibility, and separation performance. When comprehensive proteome analysis with deep coverage is needed, the 200-cm-long µPAC column, which delivers unprecedented separation performance, is an attractive option. However, the true benefit of using a 200-cm-long µPAC column will come fully into play only when it is operated with long solvent gradient times (>120 min). With an internal column volume of approximately 3 µL and an increased operational flexibility (flow rates up to 2000 nL/min), the 50-cm µPAC column serves those who are looking for increased separation performance in routine daily proteome analysis settings where shorter gradient times (<120 min) and increased throughput are desired.
Geert Van Raemdonck, PhD, is field support expert, Jeff Op de Beeck, PhD, is application manager, Natalie Van Landuyt is R&D developer, Bo Claerebout is R&D engineer, Kurt Van Mol is research engineer, Paul Jacobs, PhD, is chief operating officer, and Katrien Vanhonacker is vice president, business development, at PharmaFluidics.