![A targeted version of genomic imprinting is particularly prevalent in the brain. Left: Subset of brain cells that predominantly express a single copy of an autism-linked gene inherited from one parent (one dot). Right: Subset of brain cells that express both parents' copies (two dots). Targeted imprinting may be the preferred strategy over silencing one parental gene copy in every tissue. [Christopher Gregg, Univ of Utah]](https://genengnews.com/wp-content/uploads/2018/08/Jul31_2015_UnivofUtah_NoncanonicalGenomicImprinting2391321366-1.jpg)
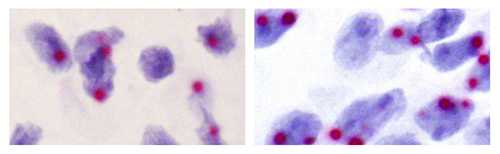
A targeted version of genomic imprinting is particularly prevalent in the brain. Left: Subset of brain cells that predominantly express a single copy of an autism-linked gene inherited from one parent (one dot). Right: Subset of brain cells that express both parents’ copies (two dots). Targeted imprinting may be the preferred strategy over silencing one parental gene copy in every tissue. [Christopher Gregg, Univ of Utah]
Genomic imprinting, a mechanism that mutes gene expression in a parent-of-origin manner, can be highly targeted, affecting not just particular tissues, but even tissue subregions, mere clusters of cells—to subtle effect. Subregion-specific imprinting in the brain, for example, has been shown to influence behavior.
This finding emerged from a study conducted at the University of Utah School of Medicine, where researchers focused on a kind of targeted genomic imprinting they call noncanonical imprinting. They used an RNA-sequencing-based approach to detect even modest maternal or paternal allele expression biases at the tissue level.
They presented their results July 30 in Cell Reports, in an article entitled, “Noncanonical Genomic Imprinting Effects in Offspring.” This article described how the scientists profiled imprinting in female mice, paying particular attention to brain subregions known to control behavior. In addition to scrutinizing these subregions, the arcuate nucleus and dorsal raphe nucleus, the researchers examined imprinting in skeletal muscle and liver.
“Using in situ hybridization for nascent RNAs, we discover that autosomal noncanonical imprinted genes with a tissue-level allele bias exhibit allele-specific expression effects in subpopulations of neurons in the brain in vivo,” wrote the authors. “We define noncanonical imprinted genes that regulate monoamine signaling and determine that these effects influence the impact of inherited mutations on offspring behavior.”
The researchers found instances of noncanonical imprinting that were common in selected brain subregions but rare, for example, in the liver. Moreover, these instances of noncanonical imprinting had behavioral effects. For example, they could motivate a timid mouse to leave its protective shelter and search for food.
The behavioral effects appeared to reflect changes in a particular biochemical pathway that is responsible for the release of serotonin and dopamine, neurochemicals that affect mood and behavior. Within this pathway, there are five genes preferably controlled by mom, or dad. The maternal copy of one of these genes, the gene for tyrosine hydroxylase, was removed by the scientists, who subsequently observed a modest but consistent increase in the amount of time the mice spent out in the open. By contrast, mice with their silenced, paternal copy removed showed no behavioral changes.
“We speculate that a better strategy for imprinting is to do it in the cells that are needed to achieve the desired effect, rather than to do it in every tissue,” said senior author Christopher Gregg, Ph.D., assistant professor of neurobiology and anatomy.
“The field has traditionally thought of genetics at the level of the whole animal, and sometimes the tissue. We're documenting it at the cellular level,” Dr. Gregg noted. “Genetics is much more complicated than we thought.”
The current study uncovered hundreds of noncanonical autosomal and X-linked imprinting effects. Of the 210 imprinted genes analyzed, 80% were subject to noncanonical imprinting, and 64% of those genes showed parental bias exclusively in the brain or subregions of the brain, and not in non-neural tissues, liver, or muscle.
Using in situ hybridization for nascent RNAs, the scientists visualized active copies of genes and determined that allele bias stemmed from differences within populations of cells. While canonically imprinted genes have just one active copy in nearly every cell examined, noncanonically imprinted genes have one active copy in subsets of cells, and two active copies in others.
The results expand on previous work by another group who found a gene that imprints in specific neurons, and is reported to be associated with autism when mutated. This and the current study's behavior experiments highlight that in addition to fine-tuning parental control, noncanonical imprinting may have a downside.
Dr. Gregg speculated that the targeted form of imprinting gives rise to “high-risk” neurons that are especially vulnerable to mutations inherited from one parent because they don't express a second, healthy back-up copy to compensate for the mistake. “We think that subpopulations of cells that preferentially express mutated genes could disproportionately contribute to brain disorders such as autism,” he explained. Future research will test the hypothesis and novel therapies to overcome the deficits.