
In 2011, Cellectis chairman and CEO André Choulika, PhD, told his employees that the Paris-based company was changing course. It was dropping the early genome editing platform that Choulika had been working on since the late 1980s, called meganucleases, and instead adopting a new technology—transcription activator-like effector nucleases, or TALENs.
It wasn’t a hard decision for Choulika, even though he had pioneered the use of meganucleases—naturally occurring enzymes found in microbes and some plants—for genome editing. “You have to be technology-agnostic because technologies are usually meant to be obsolete after a time,” Choulika says.
By mid-2012, a new gene editing technology emerged and sparked excitement among scientists, making modification of plant, animal, and even human genomes easier than ever before. That system, of course, is CRISPR-Cas9. As the hype for CRISPR built relentlessly over subsequent years, a small number of scientists, including Choulika, kept faith in older genome editing technologies: TALENs and ZFNs select zinc finger nucleases).
That perseverance is paying off.
Experimental therapies that use ZFNs and TALENs are advancing in the clinic, and TALEN-based crops are being harvested select see sidebar “Farm Aid”). The first gene edited products will be a reality soon, and they likely won’t be made with CRISPR. In the near term, companies including Cellectis and Sangamo Therapeutics in California are committed to using the older platforms, especially as safety questions around CRISPR linger. But the fate of these older gene editing platforms remains to be seen.
Even as CRISPR has exploded, Choulika hasn’t abandoned TALENs—though he did consider it. “We said, ‘Wow, that looks super cool,’” Choulika recalls when asked about his team’s first response to CRISPR.
Cellectis tried using CRISPR in early 2013, but after several months of experimentation, the company decided to return to the tried-and-tested TALENs, which it had been using to develop immunotherapies. “We found them to be more accurate, precise, and powerful, and we thought they would be safer for patients,” Choulika says.
Cellectis was awarded the first European patent for using CRISPR in T cells in 2017, but the company has no intention of pursuing regulatory approval of a CRISPR-based therapy—at least not soon. Instead, the company is focused on getting its TALEN-edited chimeric antigen receptor select CAR) T-cell therapies approved in the United States and Europe.
Early days
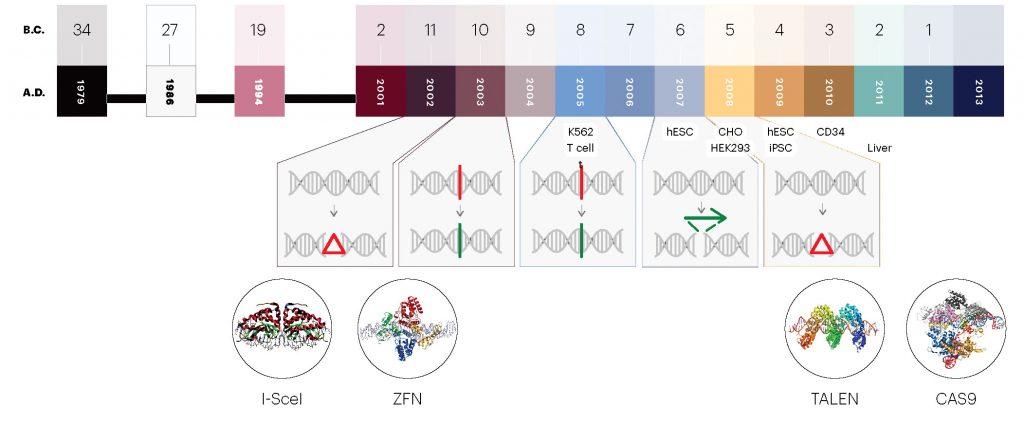
In 1994, Maria Jasin, PhD, a developmental biologist at Memorial Sloan Kettering Cancer Center in New York, was the first researcher to induce a double-strand break at a specific point in mammalian chromosomal DNA using a restriction endonuclease. “Nothing like that had ever been done before,” Jasin says. “It was such a dramatic result for me to see homologous recombination being induced so substantially.” select In a review article published last year in The CRISPR Journal, Fyodor Urnov, PhD, a pioneer of gene editing at Sangamo and now at the Altius Institute, hailed Jasin’s breakthrough as “the first key discovery in the Age of Editing.”)

Jasin’s seminal work led to interest among scientists to use endonucleases to precisely snip DNA in the genomes of complex organisms. First came meganucleases, also known as homing endonucleases, which are still used by North Carolina-based Precision Biosciences but have been largely abandoned by academic researchers because of their limited range. select Founded in 2006, Precision recently won permission from the FDA to begin a clinical trial for a gene edited, allogenic CAR T-cell therapy for B-cell acute lymphoblastic leukemia and non-Hodgkin lymphoma. It’s the company’s first clinical-stage product.)
“That platform was really what started out the whole genome editing field,” says Matthew Porteus, MD, PhD, a physician-scientist at Stanford University select and co-founder of CRISPR Therapeutics) who is developing gene editing therapies for children with genetic diseases. “But it’s been the most challenging platform in terms of trying to reengineer a protein to recognize a new target site and maintain that high on-target activity and low off-target activity.
After meganucleases came ZFNs, a class of engineered DNA-binding proteins devised by Srinivasan Chandrasegaran, PhD, a professor at Johns Hopkins Bloomberg School of Public Health, and licensed by Sangamo. Between 1994 and 2001, scientists published several proof-of-concept papers for meganucleases and ZFNs.

ZFNs are composed of a cleavage domain and typically five or six individual zinc-finger motifs, each of which can recognize three base pairs of DNA. Sangamo has stayed the course with ZFNs, which it sees as having potential advantages over CRISPR technology.
“We’ve always had a great deal of confidence in the capabilities of [ZFNs] as a therapeutic platform,” says Edward J. Rebar, PhD, chief technology officer at Sangamo. “In recent years, we’ve only gotten better at understanding them and getting better performance out of them.”
Gene editing milestones
ZFNs entered the clinic in 2009—a major milestone for gene editing. In collaboration with a group led by Carl H. June, MD, at the University of Pennsylvania, scientists from Sangamo removed T cells from HIV patients, disrupted the CCR5 gene, and infused the gene edited cells back into their bodies. It was an attempt to stop the HIV virus from infecting new cells. A few participants in the trial have been able to stay off HIV drugs since receiving the experimental treatment, but most have not.

Cellectis developed the next gene edited ex vivo therapy, a donor-derived T-cell therapy edited with TALENs, and licensed it to pharma companies Servier and Pfizer. Called UCART19, the therapy entered clinical trials in 2016, administered to two infants with aggressive B-cell acute lymphoblastic leukemia. In January 2017, researchers reported that the therapy had led to remission in both cases. But the end of 2017 brought tragic news: a patient died after receiving UCART123, another allogenic gene edited therapy developed by Cellectis.
For Sangamo, 2017 was also a landmark year. The company became the first to attempt in vivo gene editing in a patient. That November, a 44-year-old man named Brian Madeux was the first individual to receive Sangamo’s SB-913 for Hunter syndrome, or mucopolysaccharidosis type II, a rare hereditary lysosomal storage disease. The ZFN-based genome editing approach is designed to insert a corrective gene into the patient’s liver cells.
“One of the key things about our in vivo approach is that we’re using very similar zinc fingers across the board, so success in one will mean success in the others,” says Edward Conner, MD, Sangamo’s CMO.
Today’s landscape
With CRISPR all but established as the gene editing tool of choice in academia, researchers think the future of TALENs and ZFNs will likely be limited to the companies using them to commercialize products. There are already hundreds of labs and several companies using the CRISPR platform. “What’s rapidly going to happen is that the number of clinical trials using CRISPR is going to zoom past TALENs and ZFNs. It’s going to be exponential,” Porteus predicts.
Jasin thinks TALENs and ZFNs might find a role as alternatives to CRISPR if off-target effects end up being a concern for certain indications. Older gene editing technologies “could always be on the back burner for more specialized use,” she says. “If for a particular disease there’s a really well-developed zinc finger that’s not known to have off-targets, and CRISPR does, then there would be a rationale for using that.”
Porteus, whose lab raised an alarm last year about the existence of preexisting immunity in humans to Cas9 proteins, agrees. He says researchers might look to use TALENs and ZFNs again if CRISPR use in the clinic raises safety concerns. This may be less of a problem with TALENs, which originate from a type of bacteria that infects plants, and ZFNs, which are artificial enzymes to which humans haven’t been exposed.
“What if there is an unexpected event with CRISPR technology? Depending on what that adverse event is, that might rejuvenate interest in the older technologies. So I’m glad they’re there,” Porteus says.
Sangamo’s Conner sees benefits in the use of different gene editing techniques “because they have the potential to address a lot of different diseases.”
Phase II trials of UCART19 will begin later this year. Cellectis’ Choulika predicts that three of the company’s TALEN-edited cancer therapies will be on the market by 2022. Meanwhile, Sangamo is enrolling children as young as 5 years old in its Hunter syndrome trial—a testament to the therapy’s perceived safety. And last December, Sangamo announced the beginning of a Phase I/II trial for hemophilia B using a ZFN approach called SB-FIX to introduce the factor IX clotting factor gene into liver cells.
For now, TALENs and ZFNs have the early lead in the gene editing clinical race, but as several CRISPR therapies enter trials, the fate of these older gene editing platforms is uncertain. “It remains to be seen if there’s any difference between a ZFN and a TALEN and a CRISPR once you enter the clinical arena,” Porteus says.
Emily Mullin select [email protected]) is a freelance contributing writer based in Baltimore, MD.
Farm Aid
The first gene edited food product to hit the market will be one made with the TALEN system, not the CRISPR system.
Calyxt, whose parent company is Cellectis, plans to introduce its gene edited soybean oil to the United States this year. Calyxt was founded in 2010 after Choulika approached Dan Voytas, PhD, a professor of genetics and cell biology at the University of Minnesota, who was developing the first TALENs alongside Adam Bogdanove, PhD, at Iowa State University.
Until recently, soybean oil was often chemically treated through a process called hydrogenation to make it more amenable for use in cooking and to extend its shelf life. But this process gives rise to trans fatty acids, which the FDA banned in June 2018 because of a link to “bad” cholesterol and heart disease.
This negative association means consumers have gravitated toward other, healthier oils. But soybean oil is cheap to produce, and the United States is the top soybean exporter and producer in the world. In 2017, U.S. farmers grew nearly 90 million acres of the legume.
Calyxt wants to make soybean oil an attractive option for both farmers and consumers. It is making a “heart healthy” version that resembles olive oil or canola oil, using TALENs to inactivate a pair of genes in the soybean plant to change the fatty acid composition.
In fall 2017, Calyxt harvested 17,000 acres of the TALEN-edited soybean and then crushed the beans to make the oil. Voytas expects to begin selling the gene edited oil in early 2019.
“Right now, we don’t see any limitations to TALENs,” Voytas says. “We can make the types of gene edits we want to make in our crop varieties. But of course, technology advances quickly, and we’re going to have to make sure that we adapt TALENs to gene editing approaches that are going to give us products of value.” Next, Calyxt plans to roll out a high-fiber wheat edited with TALENs. The company completed a field trial in 2018 and is conducting food testing on the grain it grew.


