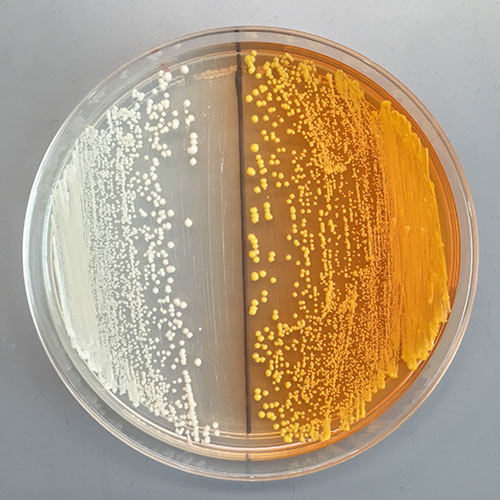
![By producing the yellow beet pigment betaxanthin, these yeast cells identified enzymes in the production of benzylisoquinoline alkaloids, poppy metabolites that can lead to morphine and other drugs. [William DeLoache/UC Berkeley]](https://genengnews.com/wp-content/uploads/2018/08/May19_2015_UCBerkeley_BetaxanthinYeastCells_HomebrewedOpiates9323619421-1.jpg)
By producing the yellow beet pigment betaxanthin, these yeast cells identified enzymes in the production of benzylisoquinoline alkaloids, poppy metabolites that can lead to morphine and other drugs. [William DeLoache/UC Berkeley]
If Walter White had been a microbrewer and not a chemistry teacher, he might have been tempted to exploit a recent advance in bioengineering. This advance, reported by scientists based at the University of California, Berkeley, makes it possible for yeast to emulate the chemistry of poppy plants and to generate metabolites such as codeine and morphine. Although all the chemical steps in the poppy’s drug pathway have yet to be engineered into a single microbial organism, doing so is within reach. Crucial steps in the pathway that had been missing, the Berkeley scientists said, can now be added.
The Berkeley scientists described their work May 18 in the journal Nature Chemical Biology, in an article entitled, “An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose.” Reticuline is a hub in a biochemical pathway that can lead to various molecules, including benzylisoquinoline alkaloids (BIAs), a diverse family of plant-specialized metabolites. BIAs can lead to the pharmaceuticals codeine and morphine and their derivatives.
From [BIA], it would be possible to explore many different paths to other potential drugs, not just opiates, the paper’s authors emphasized. For example, different paths could lead to the antispasmodic papaverine or to the antibiotic precursor dihydrosanguinarine. However, the authors also acknowledged that their discovery could make it easier for homebrewed opiates to become a reality, and they are calling for regulators and law enforcement officials to pay attention.
Homebrewed drugs from microbes would require a simple, easily obtainable input: sugar. Thus, would-be Walter Whites could avoid the difficulties of procuring tightly controlled precursors.
“What you really want to do from a fermentation perspective is to be able to feed the yeast glucose, which is a cheap sugar source, and have the yeast do all the chemical steps required downstream to make your target therapeutic drug,” said John Dueber, Ph.D., the Berkeley team’s principal investigator and an assistant professor of bioengineering. “With our study, all the steps have been described, and it’s now a matter of linking them together and scaling up the process. It’s not a trivial challenge, but it’s doable.”
But why use microbes, and not just the plants? “Plants have slow growth cycles, so it’s hard to fully explore all the possible chemicals that can be made from the BIA pathway by genetically engineering the poppy,” said study lead author William DeLoache, a UC Berkeley Ph.D. student in bioengineering. “Moving the BIA pathway to microbes dramatically reduces the cost of drug discovery. We can easily manipulate and tune the DNA of the yeast and quickly test the results.”
The Berkeley team found that by repurposing an enzyme from beets that is naturally used in the production of their vibrant pigments, they could coax yeast to convert tyrosine, an amino acid readily derived from glucose, into dopamine. Then, with help from the lab of Concordia University's Vincent Martin, Ph.D., the researchers were able to reconstitute the full seven-enzyme pathway from tyrosine to reticuline in yeast.
“[We] demonstrate the production of the key BIA intermediate (S)-reticuline from glucose in Saccharomyces cerevisiae,” wrote the authors of the Nature Chemical Biology article. “To aid in this effort, we developed an enzyme-coupled biosensor for the upstream intermediate L-3,4-dihydroxyphenylalanine (L-DOPA). Using this sensor, we identified an active tyrosine hydroxylase and improved its L-DOPA yields via PCR mutagenesis.”
“Coexpression of DOPA decarboxylase enabled what is to our knowledge the first demonstration of dopamine production from glucose in yeast,” the authors continued. “We extended this pathway to fully reconstitute the seven-enzyme pathway from L-tyrosine to (S)-reticuline.”
“We're likely looking at a timeline of a couple of years, not a decade or more, when sugar-fed yeast could reliably produce a controlled substance,” cautioned Dr. Dueber. “The time is now to think about policies to address this area of research. The field is moving surprisingly fast, and we need to be out in front so that we can mitigate the potential for abuse.”
In a commentary in Nature timed with the publication of this study, policy analysts call for urgent regulation of this new technology. They highlight the many benefits of this work, but they also point out that “individuals with access to the yeast strain and basic skills in fermentation would be able to grow the yeast using the equivalent of a homebrew kit.”
They recommend restricting engineered yeast strains to licensed facilities and to authorized researchers, noting that it would be difficult to detect and control the illicit transport of engineered yeast strains.
While such controls may help, Dr. Dueber said, “An additional concern is that once the knowledge of how to create an opiate-producing strain is out there, anyone trained in basic molecular biology could theoretically build it.”
Another target for regulation would be the companies that synthesize and sell DNA sequences. “Restrictions are already in place for sequences tied to pathogenic organisms, like smallpox,” said DeLoache. “But maybe it's time we also look at sequences for producing controlled substances.”