U.S. scientists suggest that the antidepressant fluoxetine may represent a new approach to holding back sensory and cognitive decline that occur as a natural part of aging in the brain. Research in mice by Ronen Eavri, Ph.D., and a team at the Massachusetts Institute of Technology’s (MIT) Picower Institute for Learning and Memory, found that normal brain aging is associated with a reduction in the ability of inhibitory interneurons to change and adapt, rather than with a decline in the absolute numbers of these neurons. Their studies in mice showed that this decline in neuronal plasticity in the visual cortex could be held back by treatment using fluoxetine.
“Our finding that fluoxetine treatment in aging mice can attenuate the concurrent age-related declines in interneuron structural and visual cortex functional plasticity suggests it could provide an important therapeutic approach towards mitigation of sensory and cognitive deficits associated with aging, provided it is initiated before severe network deterioration,” the authors write in their published paper in The Journal of Neuroscience, which is titled, “Interneuron simplification and loss of structural plasticity as markers of aging-related functional decline.”
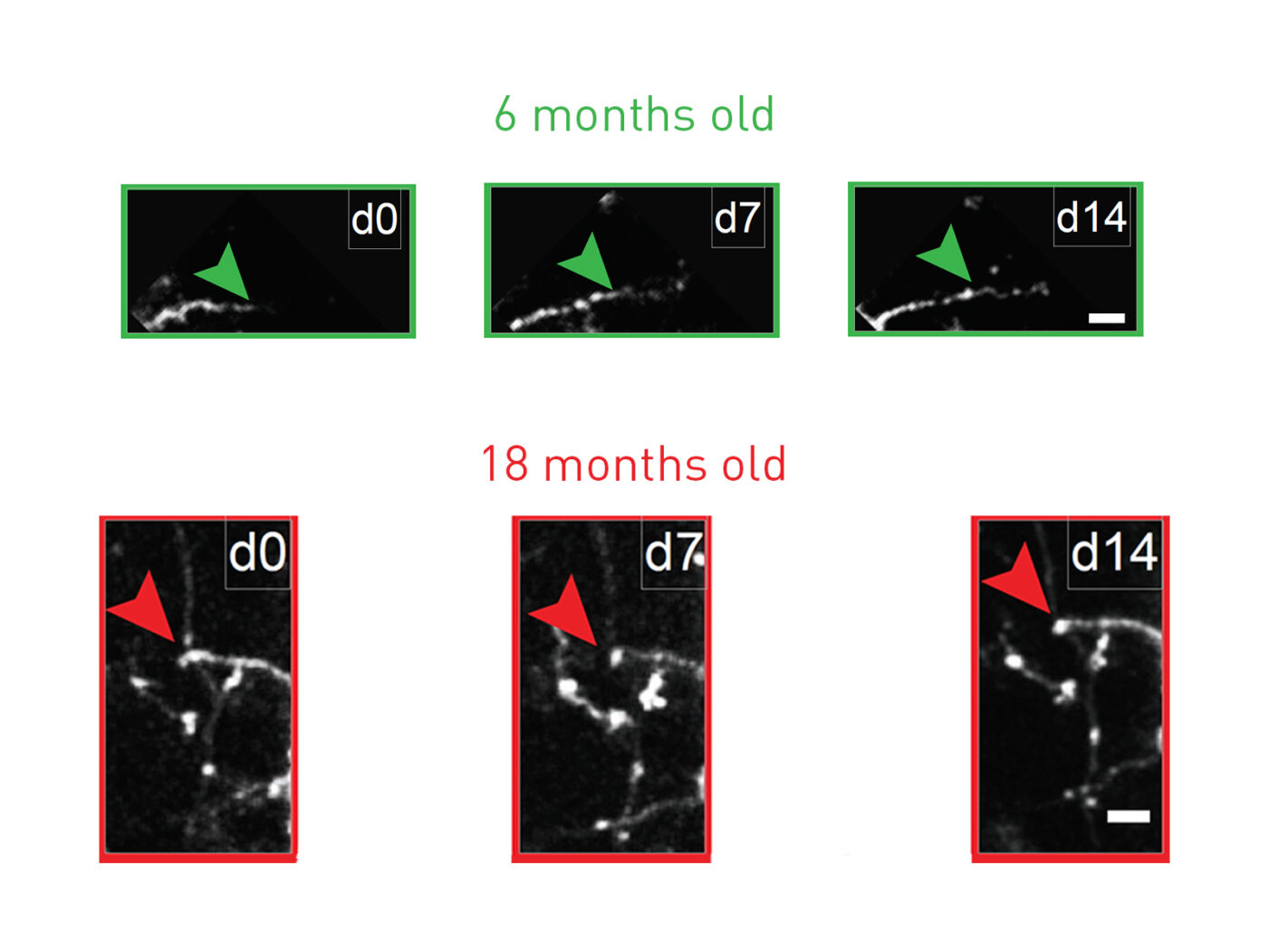
Changes in the structure of excitatory neurons in the brain are well recognized as a source of age-related cognitive decline, but far less is known about the importance of changes to inhibitory neurons. To investigate this further, the researchers studied inhibitory neurons in the visual cortex of mice, as the animals aged. Mice live for about two years. By three months of age they are mature, and by 18 months they are considered quite old. The researchers tracked the structure of inhibitory interneurons in mice aged 3, 6, 9, 12, and 18 months. What they found was that while there was no age-related change to the number of types of inhibitory neurons in the brain, their growth and plasticity starts to decline at about six months of age.
Prior work in the lab of corresponding author Elly Nedivi, Ph.D., a professor of biology and brain and cognitive sciences, MIT, had shown that inhibitory interneurons retain the ability for dynamic remodeling into adulthood. Looking at neurons under the two-photon microscope the researchers found that as the mice aged, this ability for structural change associated with remodeling declined. At an early adulthood three months of age the mice showed a balance in the growth and retraction of dendrites that connected with other neurons, a sign of dynamic remodeling. But in older mice this remodeling capacity was stunted. The inhibitory neurons put out fewer, and more simplified dendritic branches, suggestive of reduced capacity for dendritic outgrowth to connect with other neurons, in parallel with demonstrating dendrite retraction. “ … this capacity diminishes with age and is accompanied by a shift in dynamics from balanced branch additions and retractions to progressive prevalence of retractions, culminating in a dendritic arbor that is both simpler and more stable,” the authors write. There was also a critical, age-related drop in an index of dynamism.
The combined results indicated that it is the change in interneuron structure and adaptation that may be linked with cognitive decline, rather than a decline in the number of neurons “Despite common belief, loss of neurons due to cell death is quite limited during normal aging and unlikely to account for age-related functional impairments,” the team writes. “Rather it seems that structural alterations in neuronal morphology and synaptic connections are features most consistently correlated with brain age, and may be considered as the potential physical basis for the age-related decline.”
Dr. Nedivi and co-author and Picopower professor Mark Bear, Ph.D., are affiliated with MIT's Aging Brain Initiative, a multidisciplinary effort to understand how aging affects the brain. Dr. Bear’s lab also carried out a series of tests to investigate changes in plasticity that are involved in visual recognition memory in the visual cortex, through which neurons respond more intensely to stimuli to which they have previously been exposed. These results showed that while mice aged three months demonstrated robust stimulus-selective response potentiation (SRP), equivalent measures of SRP declined through six months and nine months, in parallel with reduced structural plasticity. “Recording of visually evoked potentials (VEPs) shows that aging-related interneuron dendritic arbor simplification and reduced dynamics go hand in hand with loss of induced SRP, a paradigm for adult visual cortical plasticity,” the authors write.
The question is, can this reduced plasticity be reversed or held back? Previous work by Dr. Nedivi’s lab had shown that fluoxetine can promote interneuron branch remodeling in young mice, so they tested the drug’s effects on neuronal plasticity in aging animals. Animals at different ages were given the drug in their drinking water, for different periods of time. The results showed that fluoxetine administration had little effect on dendrite growth when therapy was started at age three months and continued until they reached six months In contrast, when the treatment period was extended to six months, the researchers found that 67% of the neurons showed new growth by the time the animals reached nine months of age. And even when fluoxetine treatment wasn't started until the animals reached six months of age, the drug was still associated with 25% of cells demonstrating new growth.
Administering fluoxetine therapy for six months also significantly restored SRP, whereas the effect was much less when treatment was only maintained for three months. “Chronic treatment with the antidepressant fluoxetine reversed deficits in interneuron structural dynamics and restored SRP in aged animals,” the authors write. “Our results support a structural basis for age-related impairments in sensory perception, and suggest that declines in inhibitory neuron structural plasticity during aging contribute to reduced functional plasticity.”






