Accumulation of fat in the liver (nonalcoholic fatty liver disease, or NAFLD), compounded by cellular damage and inflammation (nonalcoholic steatohepatitis, or NASH) increases the risk for liver cancer (hepatocellular carcinoma). Cellular stress generated by unhealthy diets and pathological conditions prompts cells to go into a state where they no longer divide (cellular senescence) but continue to secrete a plethora of harmful factors that degrade proteins and promote inflammation and cancer. Together these secreted factors constitute a “senescence-associated secretory phenotype,” or SASP.
Earlier studies have focused on mechanisms that induce SASP but little is known about how SASPs are released by cells and cause cancer. A study in the journal Science Immunology, titled, “Gasdermin D–mediated release of IL-33 from senescent hepatic stellate cells promotes obesity-associated hepatocellular carcinoma,” uses a mouse model of obesity-induced liver cancer to show how aging liver fibroblast cells (hepatic stellate cells) release a tumor-promoting SASP factor called interlukin-33 (IL-33) through pores in the liver cell membrane produced by a membrane-spanning protein called gasdermin D. This mechanistic insight points out the therapeutic potential of inhibiting gasdermin D mediated pore formation in treating liver cancer.
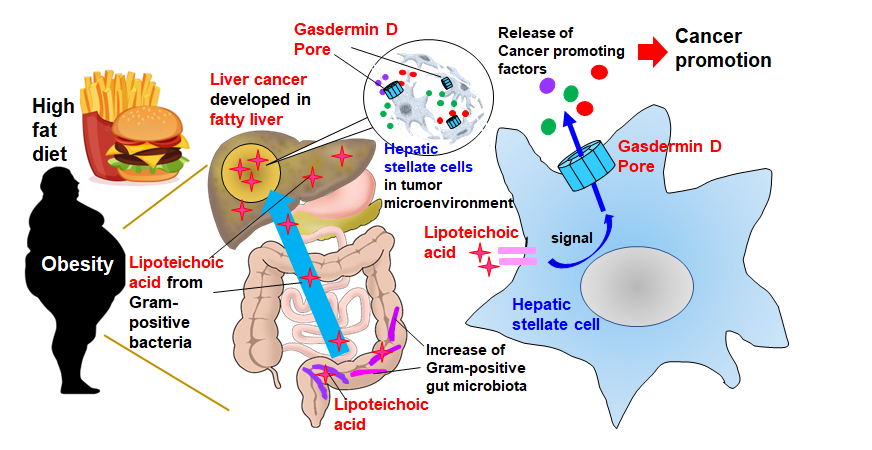
“In the obesity-associated liver tumor microenvironment, the fibroblasts called ‘hepatic stellate cells’ become senescent,” explained Ohtani. “This causes them to exhibit a SASP, where they release a set of proteins that promote cancer by suppressing anti-tumor immunity.”
The investigators fed cancer-prone mice a high-fat diet and conducted a comprehensive gene expression profiling study to analyze SASPs produced by hepatic stellate cells in mice with obesity-induced liver cancer. They found the SASP factors IL-1β and IL-1β-dependent IL-33, are the primary culprits that are released from liver fibroblasts and promote cancer.
“First, the high-fat diet weakens gut barrier function, resulting in the migration and accumulation of lipoteichoic acid in the liver,” explained Ohtani. “Second, the accumulated lipoteichoic acid stimulates the cleavage of gasdermin D protein. This, in turn, forms cell membrane pores through which IL-1β and IL-33 are exported or released from hepatic stellate cells.” Lipoteichoic acid is a cell wall component of Gram-positive bacteria.
Once IL-33 is released, it activates regulatory T cells that act to suppress immune attacks on cancer cells and potentially exacerbate cancer development. Deciphering the mechanism of SASP release, therefore, is a key advancement in developing therapies against liver cancer.


