By Priya Kalia, PhD
If modern cell-based bioprocessing is to improve its ability to churn out biotherapeutics—not just tried-and-true antibodies, but also bi- and trispecific antibodies, as well as viral antigens and other novel vaccines—it will have to address certain upstream manufacturing challenges. At their core, these challenges are all about—you guessed it—the cells. Sometimes, the cells just need better accommodations and a bit of pampering. At other times, they might perform better if they undergo a little tinkering or even an extensive overhaul.
Options such as these will be discussed at the 17th annual Cell Culture & Cell Line Optimization conference, which is scheduled to be held August 16–17. A preview of this event is available in this GEN supplement article. Several of the event’s presenters were approached by GEN, and they responded by describing their latest work and sharing their thoughts on the industry.
We present the scientists’ comments here. They range from clone selection technologies (such as omics, optofluidics, and siRNA) to continuous monitoring. Some comments even reflect a willingness to look beyond cells, that is, to the potential for cell-free manufacturing—an increasingly attractive production mode, given the rise of mRNA therapeutics.
Overcoming rate-limiting steps
“Cell line development is still one of the rate-limiting steps in the bioprocessing end of bringing a new drug to market,” said Susan Sharfstein, PhD, professor of nanobioscience, SUNY Polytechnic Institute. “Some of the biggest challenges include clone stability and the production of molecules that nature never intended, like bispecific antibodies.”
These challenges, Sharfstein remarked, are certainly relevant to the development of Chinese hamster ovary (CHO) cell lines. CHO cell lines were established in the 1950s, and they have become the mammalian cell lines of choice. In fact, they are behind the manufacture of about 70% of biotherapeutics. They provide diverse, correctly folded, and properly glycosylated proteins with human-compatible post-translational modifications. They also offer high productivity and adaptability to large-scale culture with chemically defined media.
“There is an inherent genetic instability and variability with CHO cells,” Sharfstein emphasized. “[This] has great advantages for the industry—as well as certain disadvantages.”
A similar point was offered by Renee Tobias, director of CLD Product Management at Berkeley Lights. “Highly engineered proteins produced in cell lines for biotherapeutic or diagnostic purposes are prone to quality issues such as aggregation,” she said. “[This tendency has] implications for drug manufacturability, shelf life, and patient safety.”
One of the essential first steps in biotherapeutic production is clone selection. To select the right clones for expressing the target protein, the industry still relies on a conventional technique—the expression of an antibiotic resistance gene and the use of antibiotics such as neomycin or dihydrofolate reductase (DHFR). However, highly efficient alternatives to this technique might be available soon, thanks to research conducted by Sharfstein and her collaborator Scott Tenenbaum, PhD, associate professor of nanobioscience at SUNY Polytechnic Institute and head of nanobioscience at the College of Nanoscale Sciences and Engineering at SUNY Albany. (He is also CSO at sxRNA Technologies.)
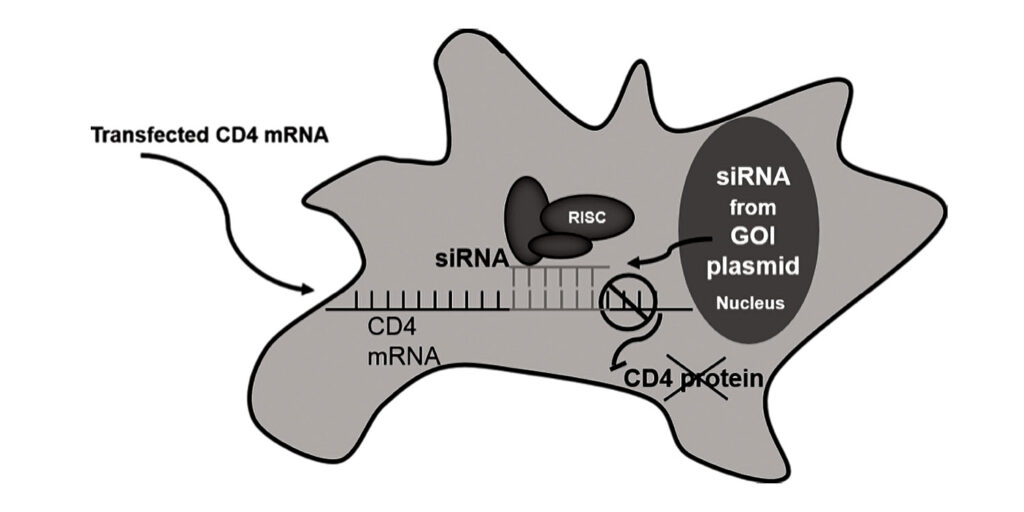
The research has already enabled Sharfstein and Tenenbaum to devise a new clone selection method. In this method, an expression vector allows the gene of interest to be co-transcribed with a noncoding RNA (such as small interfering RNA, or siRNA). An mRNA that codes for cell surface marker—for example, CD4, the gene sequence for which is interrupted in the middle with a 3′ untranslated region complementary to the siRNA—is then transiently transfected into the cells.
When the siRNA is transcribed (along with the gene of interest), the 3′ untranslated region interrupting the CD4 mRNA pairs with the transcribed siRNA and is subsequently degraded, resulting in an uninterrupted, translatable CD4 mRNA transcript. Once CD4 is translated, it can be expressed on the cell surface, and cells can then be selected using this marker. Advantages include transient expression of CD4, the method’s speed compared with traditional methods, and the fact that antibiotics are not required.
“We also get a much larger fraction of clones positive for expression of the gene of interest than with traditional approaches,” Sharfstein asserted. Tenenbaum added that the use of proxy genes, such as antibiotic resistance genes, has persisted because the industry has continued to use methods that work well enough and have regulatory approval—although it is now “fairly antiquated.”
Applying omics and optofluidics
Another exciting development that is helping to evolve clone selection is the harnessing of omics and bioinformatic analysis. Sofie O’Brien, PhD, scientist, BioProcess and Analytical Sciences, Seagen, says that her team’s work is focused on bioinformatics and how it can be used with CHO cell line development, specifically clone selection, to gain a better understanding of transcriptional patterns. The team hopes that a bioinformatics approach could make it easier to identify where transgenes integrate.
Seagen is developing technology that could accelerate the stability studies that are performed with new clones. Such studies are time and resource intensive, and they often prove to be rate limiting for cell line development.
“By applying omics, we are using a more holistic approach to cell line development,” commented O’Brien. “[This approach give us] a more complete understanding of the factors influencing our cell lines, potentially saving time and resources.”
At Berkeley Lights, Tobias and her team are focusing on optofluidics for clone selection. By targeting the early stages of clone selection, the scientists hope to minimize the risk of late-stage failure and to ensure that the right quantity and quality of the desired product are achieved.

“With the Beacon system, thousands of cells can be cloned and assayed in parallel at the microscale, reducing expansion times and plate handling steps with 1/30th the labor of traditional clone selection workflows,” Tobias asserted. “Our technology has therefore made significant impact on the development of vaccines and antibodies against SARS-CoV-2.”
This includes work with the research team led by Trent Munro, PhD, professor and senior group leader, Australian Institute for Bioengineering and Nanotechnology, University of Queensland. Munro and colleagues were able to expedite a process that incorporates cell line development and generates a recombinant protein vaccine. The scientists showed that the process, which typically takes years, could be completed in 78 days—from initiating cell processing to dosing patients.
Another example that Tobias cited involved the laboratory led by James E. Crowe, Jr., MD, at the Vanderbilt University Medical Center. When the laboratory applied Berkeley Lights’ Opto™ Plasma B Discovery workflow on its Beacon optofluidic system, it was able to find antibodies against SARS-CoV-2 more quickly. The development time needed to deliver manufacturing-ready antibodies was reduced from 24 months to within 25 days. This result, which was achieved during a project to deliver antibodies to AstraZeneca for a current Phase III clinical trial, demonstrates the advantage of focusing on achieving the right clones early on.
A role for continuous monitoring
Once the right clone has been selected and expanded, ensuring large-scale cultures are healthy and viable is important for consistent protein expression. Commonly used assays for measuring cellular health, including legacy stain Trypan Blue, provide a snapshot but are not always completely accurate.
“Trypan Blue has been used for over a century as a measure of cell viability, when in reality it is a measure of the loss of cell membrane integrity,” remarked Michael Butler, PhD, principal investigator, Cell Technology Group, National Institute for Bioprocessing Research and Training (NIBRT), Ireland. “The challenge is to convince the industry that this may not be the best measure of cell viability.”
His group has been studying the use of optics and dielectrics to monitor mammalian cells and develop an automated continuous monitoring system for bioprocesses. Butler emphasizes the subtlety that this approach provides.
“It’s important to bear in mind that early cell death events do not affect the integrity of the cell membrane,” he elaborates. “It can therefore remain intact—something that Trypan Blue would not detect. This happens further along the pathway.
“Applying more sensitive, continuous monitoring to recombinant protein production would allow greater monitoring of metabolic changes in the cell, and it could allow the option of terminating the culture at an earlier stage, before losing membrane integrity—potentially lessening the contamination of a desired recombinant protein with host cell proteins.”
Cell-free mRNA manufacturing
As highly valued as antibodies and other proteins have become as therapeutic modalities, they could become even more valuable—if only they could be produced more efficiently. It is this possibility that has motivated the development of the technologies described in this article thus far.
Although these technologies promise to improve cell culture and cell line development, and thereby ease the production of antibodies and other proteins, they may not be ideally suited to the production of other biologics, including biologics of the sort that have received significant international attention throughout the COVID-19 pandemic.
In the popular imagination, these biologics are exemplified by the Pfizer-BioNTech vaccine and the Moderna vaccine against SARS-CoV-2. These biologics, of course, are mRNA-based products. Their upstream manufacture does not require cell culture. Instead, their manufacture can be achieved via cell-free manufacturing, avoiding multiple challenges faced by cell-based therapies.

“In principle, mRNA is not a new technology, although during the pandemic, the world has woken up to its many advantages,” said Andreas Kuhn, PhD, senior vice president, RNA Biochemistry and Manufacturing, BioNTech. According to Kuhn, these advantages include greater manufacturing flexibility and easier scale-up than are possible with cell-based platforms.
“The mRNA manufacturing process is a highly scalable and robust process,” he continued. “It can be standardized and does not need to be adapted for each new vaccine. The very same process can also be used to manufacture individualized cancer vaccine candidates.
“Another advantage is that manufacturing cycles are relatively short. The entire manufacturing process technically takes only about two weeks and can be flexibly adapted to a new mRNA sequence. It is possible, for example, to react faster to virus variants of concern, which makes mRNA an ideal way to address emerging pandemic threats such as COVID-19.”
The manufacture of mRNA relies on an enzymatic cell-free process in which DNA is used as a template in combination with an RNA polymerase. Of course, the enzymes required for this process are produced by cultures, albeit microbial ones.
“The cell culture footprint associated with our microbially sourced starting materials is smaller than that associated with biotherapeutics requiring mammalian cell culture,” Kuhn commented. “The manufacturing of the latter is by nature more complicated and laborious than the manufacturing of microbially derived enzymes in terms of process, time, and cost.”
Kuhn explained that because the cell-free manufacturing of mRNA is still new, starting and raw materials of appropriate quality still come with a significant price tag. However, he anticipates that prices will fall as mRNA manufacturing output grows to supply the many billions of mRNA-based vaccine doses that will be demanded in the coming months.
Given that cell-free bioprocessing offers such large advantages over mammalian cell-based bioprocessing, is the future of biological therapies and vaccines mRNA-based? “We still have much to learn, with many different applications of this technology being investigated internationally, and with challenges related to mRNA-based vaccines and/or biotherapeutic storage,” Kuhn stated. “Ultimately, I think it is likely that mRNA-based therapies and vaccines will have a place among cell-based therapies such as antibodies and cell therapies as well as viral vectors, with some modalities more suited to certain applications and/or disease areas than others. At BioNTech, for example, we are researching four complementary drug classes—mRNA is only one of them. The others are T-cell therapies, antibodies, and small-molecule immunomodulators.”
The experts agree that biotherapeutic manufacturing will continue to rely on mammalian cell culture and cell line development. The experts also suggest that these production modes will inspire innovation and technological advances as long as they enable the efficient manufacture of high-quality biotherapeutics that benefit patients and address clinical needs.






