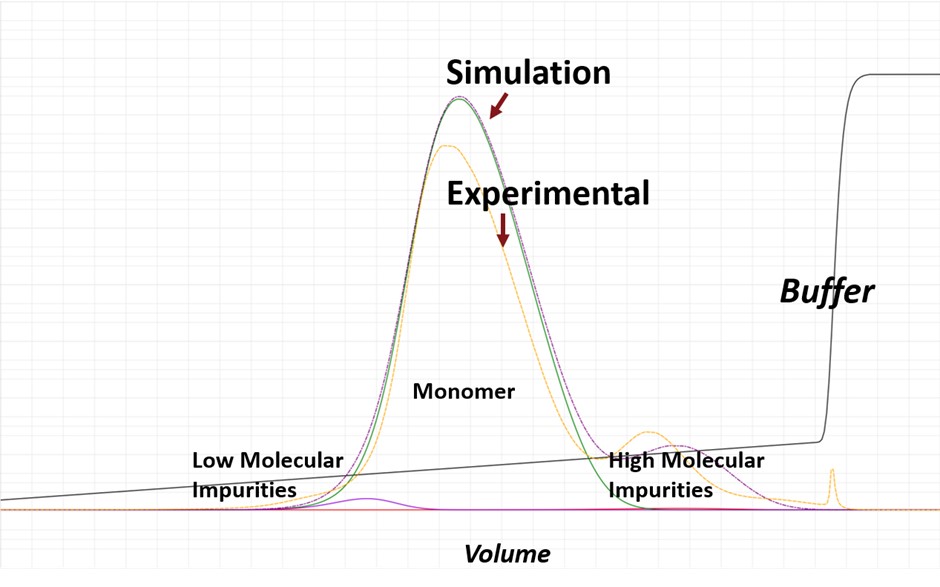
A process model designed by Bolt Biotherapeutics using the GoSilico ChromX simulation software not only did more experiments in 24 hours than a physical lab, but also reduced the amount of materials needed.
“The reason we chose to do this modeling is that we saw the true benefits of it,” explains Yuyi Shen, PhD, Bolt’s associate director of process development and manufacturing. “It’s not a showcase. It’s not just beautiful, futuristic technology–it’s practical, it’s applicable to process development, and the results matter.”
According to Shen, the software model can run 2,000 to 5,000 process modeling experiments overnight, compared to three physical experiments in 24 hours. In addition, the computer model doesn’t require massive material generation from high-titer production of a biotherapeutic to feed into the chromatography columns to establish the modeling calibration.
“Upstream, you might need multiple large-scale bioreactor runs just to generate enough material for physical experiments required to define the process parameters,” says Shen. “It saves on the cost of material generation, it saves time, and you understand the molecule better.” In addition, she adds, it helps some early-stage companies to maximize the return of their capital investments.
The company is using the software to accelerate process development of its BoltbodyTM immune stimulating antibody conjugates. The model uses experimental data about the drug candidate, as well as the dynamic mechanics of the chromatography resin, to make predictions about the biotherapeutic yield and purity.
According to Shen, it’s been particularly useful during the COVID-19 pandemic. “It’s an adaptive tool for the fast-paced biotech industry,” she says. “And, this year, when we’ve been working from home a lot, it’s a good tool to design experiments and gain critical knowledge from day one remotely.”
The model itself is specifically tailored to Bolt’s drug candidates. However, according to Nathan Ihle, PhD, Bolt’s vice president for chemistry, manufacturing, and controls (CMC) & quality, the approach can be applied to multiple targets, multiple molecules, and at a variety of stages in bioprocess design.