Sponsored content brought to you by

In order to understand biology, it is critical to be able to study the heterogeneity within a population of cells, in their tissue context. Only then can the diversity of tissues and organs be explored.
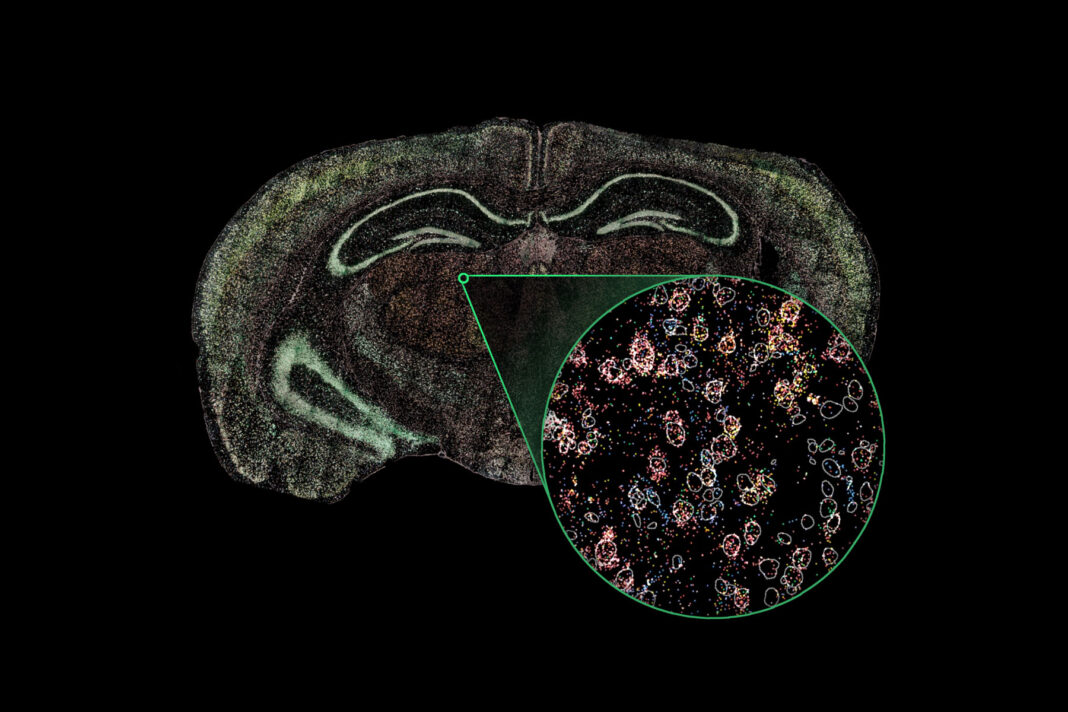
But this kind of study poses a challenge. “How do we understand the minute differences between individual cells that are next to each other while simultaneously understanding things at the broad, anatomical level?” asks Josh Ryu, PhD, co-founder and CTO at Rebus Biosystems.
Overcoming optical obstacles
As Ryu explains, in conventional optical microscopy, high resolution and large scale are not easy to achieve simultaneously.
“The resolution of a microscope is usually dependent on magnification, and inversely linked to performance metrics like field of view, working distance, and depth of field,” Ryu says. “This forces users to make a compromise between seeing the subcellular details of cells and seeing many cells at once.”
In his doctoral research at MIT, Ryu and his colleagues sought to expand the boundaries of biology by developing an imaging technique that could decouple resolution from other key lens performance metrics. Their research led to Synthetic Aperture Optics, or SAO, which is now at the heart of his company’s Rebus Esper spatial omics platform.
SAO relies upon light patterns created by interfering lasers arranged around a 20× air objective. A series of images is captured and then automatically reconstructed using proprietary algorithms to generate a single high-resolution image with resolution and sensitivity equivalent to an image captured with a 100× oil-immersion lens.
“With SAO, there’s no longer any need to compromise. You get resolution and scale,” Ryu explained. “Visualization of subcellular detail is made possible while maintaining the advantages of a 20× lens.”
Advanced optics meet ease of use
To enable more scientists to benefit from SAO, the team at Rebus Biosystems engineered a completely automated platform for spatial analysis around these advanced optics.
“Our fluidics technology delivers reagents using the optimal combination of flow rate and temperature,” Ryu says. “This enables precise reaction control, allowing robust results across tissue types with exceptional speed.”
To analyze a sample using the Rebus Esper, a scientist simply sections a sample to a coverslip and installs it into the fluidics flow cell. The flow cell is then loaded into the instrument along with Rebus Biosystems–provided reagents. The first assay to be released is based on high-sensitivity, high-specificity cyclic single-molecule fluorescence in situ hybridization (smFISH) chemistry.
“After loading the sample and reagents and using our simple software to define a region of interest, the user pushes a button to start the run and then walks away,” Ryu says. “There is no molecular biology that the user needs to do. Our platform allows biologists to focus on biology, not technology.”
After the run is complete, the Esper Spatial Studio software suite processes the collected data and allows exploration.
“The software takes the high-resolution imaging data and processes it into a format you can use directly for single-cell analysis and spatial mapping,” says Ryu.
Ryu envisions many scientific advances ahead for users of this technology. “The functions of cells are determined by multiple types of molecules,” he says. “With the Rebus Esper, we will eventually detect all of these molecules at high resolution and put them in the context of their interactions and the overall morphology of cells and tissues. Integrating all of this information will truly be the key that unlocks the mysteries of biology.”
To learn more, visit: rebusbio.com