Sponsored content brought to you by
From vaccines to therapeutics, the emergence of mRNA as a versatile and potent biological tool across the healthcare landscape continues to demand new, innovative ways to scale mRNA manufacturing. To successfully produce synthetic mRNA for therapeutic applications, linear RNA molecules must be modified with 5’-cap structures to enable effective translation into protein, and to avoid triggering the innate cellular immune system.
During manufacturing of therapeutic RNA, mRNA 5’-cap structures are added by introducing chemically synthesized mRNA cap analogs during transcription, or by enzymatic processing of RNA transcripts. The introduction of New England Biolabs’ (NEB’s) Faustovirus Capping Enzyme (FCE) delivers new and enabling capabilities for mRNA synthesis workflows.
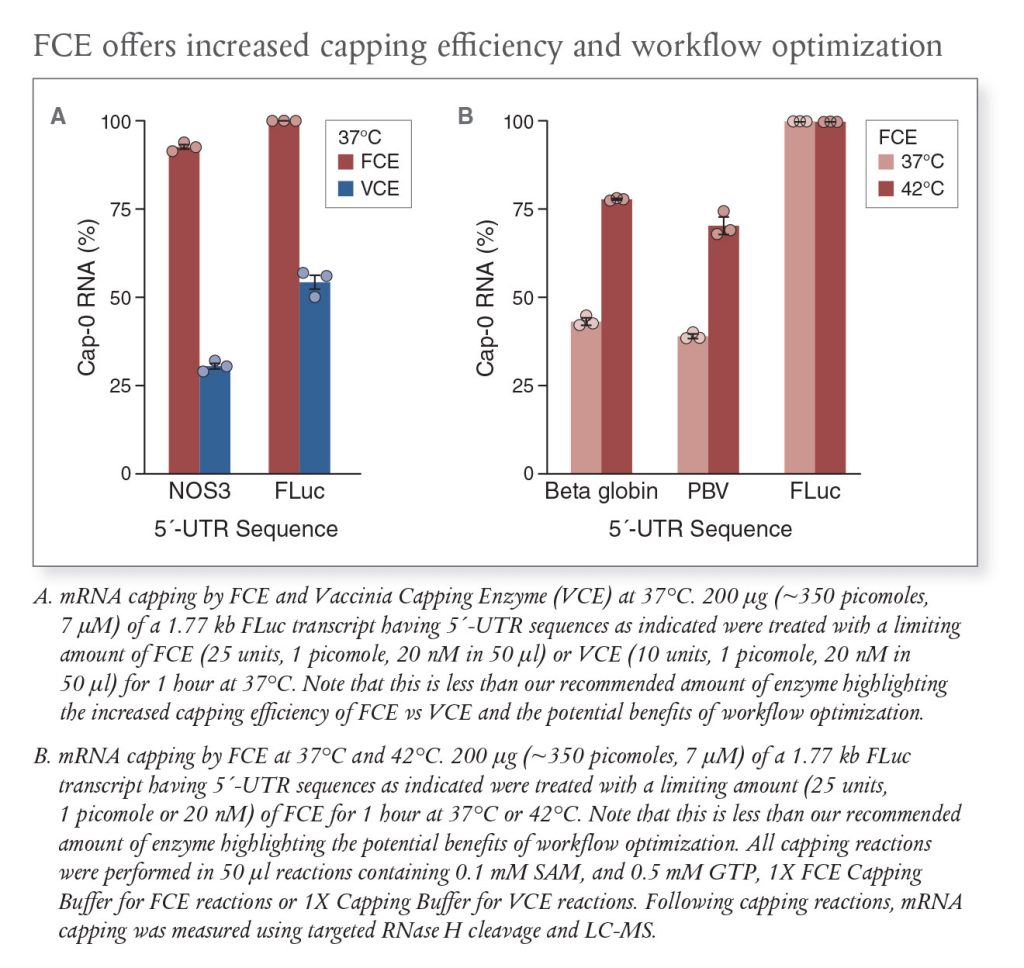
Discovered and developed at NEB with mRNA manufacturing in mind, FCE is a single polypeptide enzyme with ~2.5-fold higher specific activity compared with Vaccinia Capping Enzyme (VCE). These features bring forth higher overall yield of capped mRNA, opportunities to reduce the amount of enzyme required in scaled workflows, and the potential for decreased residual protein in mRNA products. The higher activity of FCE also results in greater capping efficiency on difficult-to-cap mRNAs. Further, when used alongside NEB’s mRNA 2’-O-methyltransferase for one-pot Cap-1 synthesis, the two enzymes provide a one-step solution for Cap-1 modification.
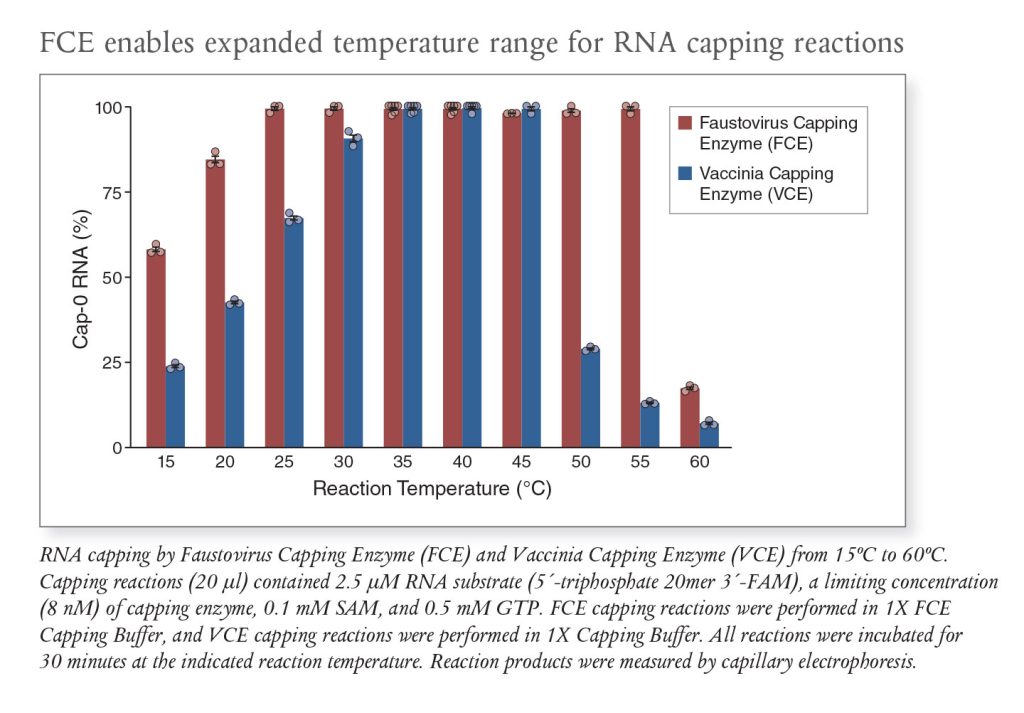
Additionally, FCE functions over a broad temperature range (20°–55°C), alleviating restrictive temperature constraints. From a production and engineering perspective, a broader effective temperature range creates potential manufacturing benefits—particularly for enabling processes requiring higher temperatures needed to cap difficult substrates. Alternatively, lower temperatures can be used when mRNA stability and reaction temperature regulation are of concern.
Together, the improved properties of FCE can provide substantial benefits for, and potentially remove current bottlenecks from, large-scale mRNA manufacturing workflows.
To learn more visit www.neb.com/m2081






