By Silke Wissing,* Helmut Kewes,* and Nico Scheer*#
Vaccination is undoubtedly one of the most effective means of preventing infectious diseases by provoking an active acquired immunity to pathogens, such as harmful bacteria or viruses. This is particularly evident in countries with active vaccination programs where devastating diseases like diphtheria or polio have essentially been eradicated.1 The WHO estimates that 2.3 million lives are saved each year by current immunization programs.2
As vaccines are administered prophylactically to healthy individuals, they need to fulfill particularly high demands. They must have an extremely favorable safety profile, and they should be highly effective. If intended for worldwide use, they must be inexpensive. They should also be thermostable (to avoid the need for an expensive cooling chain) and easy to administer (to minimize the requirements for specially trained personnel).
Conventional vaccines are primarily based on live attenuated pathogens, inactivated viruses, or protein subunits. Recent advances have led to promising and viable alternatives, such as virus-like particle-, DNA-, mRNA-, and viral-vector-based vaccines. The latter two approaches have received particular attention due to the approval of effective COVID-19 vaccines in the Corona pandemic, impressively demonstrating their suitability for rapidly combating emerging pathogens.3,4 Recombinant adenoviruses (Ad), which were used for the vector-based COVID-19 vaccines Vaxzevria and Janssen COVID-19 Vaccine, have additionally demonstrated their potential with the approval of the Ebola vaccine Ad26.ZEBOV in 2019.5
Among the specific advantages of Ad-based vectors are their cost-effectiveness and thermostability.6 Furthermore, these vectors can elicit a strong humoral and cellular immune response, and they stimulate both the innate and adaptive arms of the immune system. They efficiently infect various proliferating and quiescent cell types, allowing their use for systemic and mucosal routes of administration. Finally, using appropriate cells, adenoviral vectors can be produced very economically under GMP conditions. In short, Ad-based vectors are appealing tools for vaccine development due to their safety, efficacy, and ease of manufacturing.
While there are good reasons for using Ad-based vaccines, there are certain details to consider. In particular, these vaccines rely on replication-defective adenoviruses which, after infecting a target cell and transiently introducing their expression cassette for the antigen, cannot replicate and spread further in the body of the vaccinated person. This is important because replicating adenoviruses can trigger pathogenic processes and adverse immune responses.7,8 Accordingly, for Ad-based vaccines, the genome of the adenovirus has been modified so that regions which are essential for virus replication are deleted. The most common of such vectors are deficient for the Ad E1 region, so-called Ad∆E1 vectors. In order to manufacture these vectors, specific producer cell lines are required that have stably integrated the E1 region into their genome and can thus provide the E1 functions in trans. A well-known example of such cell lines is the human embryonic kidney cell line HEK293.9 However, due to the special constellation of the E1 sequence integrated into the genome of HEK293 cells, there are relatively long homology regions to the commonly used Ad∆E1 vectors. As a result, homologous recombination can occur with a certain probability, which has been shown to lead to the formation of replication-competent adenoviruses (RCAs).10 Importantly, FDA guidelines restrict their appearance to less than 1 RCA in 3 x 1010 virus particles.11
To overcome this problem, the CAP® cells were developed, which also trans-complement the E1 function. In contrast to other cell lines, the sequences integrated into the genome of CAP® cells were designed so that large homology regions were avoided.12 Therefore, RCA formation in CAP® cells is highly unlikely.13 The CAP® Ad platform also fulfills additional important criteria for a desirable production system. For example, it is capable of producing Ad vectors at high titers. CAP® cells are of non-tumor and ethically acceptable origin, as they are derived from routine amniocentesis material. Moreover, they grow in single-cell suspension to high cell densities using serum-free, chemically defined media, and they are compatible with all common bioreactors allowing an easy scale-up to large volumes. Furthermore, GMP-compliant materials and protocols are available, and a Biological Master File was deposited for reference with the FDA. Therefore, CAP® Ad represents a uniquely suitable platform for industrial-scale manufacturing of Ad-based vaccines (Figure 1).14
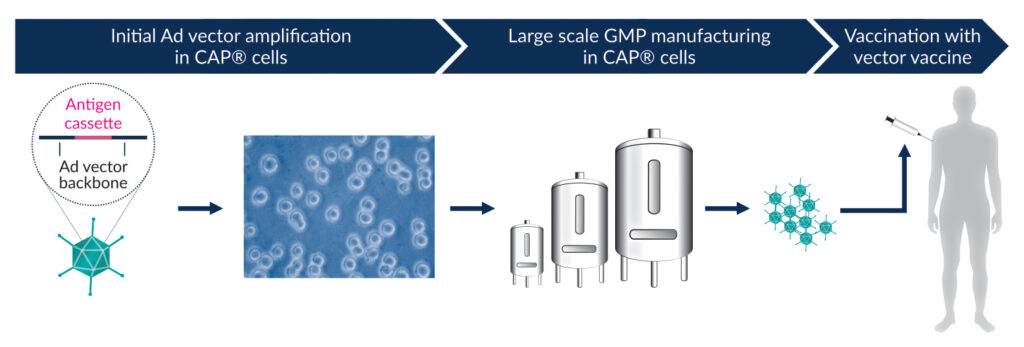
*CEVEC Pharmaceuticals, Cologne, Germany; #FH Aachen, University of Applied Sciences, Germany
References
- www.who.int/gho/child_health/mortality/mortality_under_five_text/en/ (2020).
- www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/ (2013).
- www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines
- www.ema.europa.eu/en/human-regulatory/overview/publichealth-threats/coronavirus-disease-covid-19/treatmentsvaccines/vaccines-covid-19/covid-19-vaccines-authorised
- www.ema.europa.eu/en/news/new-vaccine-preventionebola-virus-disease-recommended-approval-european-union
- Holm et al., Vaccine 39, 457–459 (2021)
- Lochmüller, H. et al., Hum Gene Ther. 5(12) (1994)
- Hermens, W.T., Verhaagen, J. Prog Neurobiol. 55(4) (1998)
- Graham, F.L. et al., J Gen Virol. 36(1) (1977)
- Murakami, P. et al., Hum Gene Ther. 13(8) (2002)
- Kovesdi, I., Hedley, S.J. et al., Viruses 2(8) (2010)
- Schiedner, G. et al., Hum Gene Ther. 11(15) (2000)
- Wissing, S. et al., GeneticEng. (35) (Oct.2021)
- Wissing, S. et al., BioProcess Int. (Oct.2021)
Learn more at www.CEVEC.com





