August 1, 2016 (Vol. 36, No. 14)
Anne Hammerstein Scientist TTP Labtech
A Simple, Safe, and Robust Approach to Screening Using Fluorescence Cytometry
Human respiratory syncytial virus (RSV) is a syncytial virus that can cause serious respiratory tract infections, especially in infants and young children.1 To date there is no licensed vaccine against RSV infection and the only preventive treatment option is the neutralizing antibody palivizumab, which is given to high-risk infants by passive administration.2 Therefore there is a strong drive to identify novel RSV-neutralizing antibodies for therapeutic/preventative applications.
Here we present the development and implementation of a simple RSV-neutralization assay on TTP Labtech’s mirrorball fluorescence cytometer. Compared to established virus neutralization assays, this method offers several distinct advantages:
- Homogeneous, no-wash protocol captures data from adherent and detached cells
- Cells and virus remain safely contained within the assay plate with mirrorball’s laser scanning approach
- Compatible with high-density 96-, 384-, and 1,536-well plates
- Whole-well scanning delivers robust data for uneven cell distribution
- Assay readout normalized to total cell count
- Mirrorball’s in situ image-based read preserves cell morphology, highlighting the fused-cell phenotype of infected cells
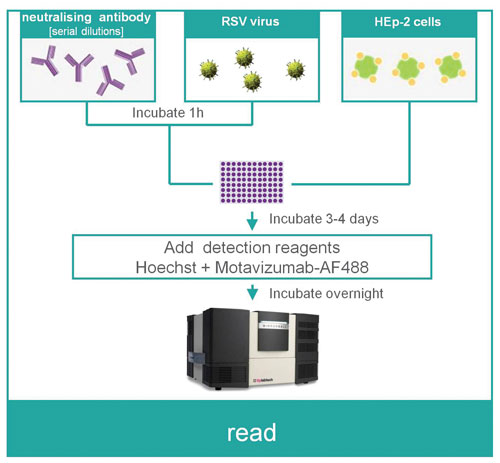
Figure 1. Method outline for the RSV neutralization assay
Materials and Methods
Materials
- HEp-2 cells (2,000 cells/20 μL, single cell suspension)
- RSV virus (500 pfu/10 μL)
- Neutralizing mAB
- Cell culture medium
- 384-well assay plate (Corning #3712)
- Detection antibody (motavizumab, conjugated to AF-488), Figure 1
Methods
Day 1
- Prepare serial dilutions of the neutralizing mAB in cell culture medium
- To each well of the assay plate add 10 μL of RSV virus and 10 μL of the neutralizing antibody
- Incubate plate for 1 hour at 37°C
- Add 20 μL of the HEp-2 cell suspension to plate and incubate for 4 days to allow the infection to proceed
Day 4
- Add 10 >μL of motavizumab-AF488 detection antibody (4 μg/mL, final concentration = 0.8 μg/mL) and Hoechst (2×) to the plate and incubate overnight to stain
Day 5
- Read the plate on the mirrorball instrument
Results
The neutralization of RSV before exposure to HEp-2 cells was assessed by a homogeneous immunofluorescence assay in 384-well plates. Whole-well images (Figure 2) immediately highlight that the number of RSV-infected green fluorescent cells increases with decreasing concentration of the neutralizing antibody. This increase is accompanied by a change in cell morphology: whereas the noninfected cells grow as a disperse monolayer with distinct nuclei for each cell, the infected cells show clear signs of multinucleation (syncytia formation), one of the hallmarks of RSV infection.3
Several readouts may be used to quantify RSV infection.
By considering the total fluorescence intensity of cells in the green channel (FL-2), the total number of infected cells can be estimated.
The concentration-response curve (Figure 3A) shows a good fit to the datapoints at high concentrations of the neutralizing antibody. However, the fit is less representative at lower concentrations. At the lowest concentration of the neutralizing antibody there appears to be a decrease in the number of infected cells. In isolation, this result would suggest that the assay was unreliable; however this is not the case.
RSV infection is known to inhibit cell proliferation, so a better readout for this assay should consider the proportion of infected cells, represented by the readout “ratio of total green (FL-2) intensity: total blue (FL-1) intensity.” The concentration-response curve for this readout (Figure 3B) now shows an excellent fit to the datapoints across the whole range. Finally, by considering the median area of nuclei in the well (Figure 3C), mirrorball can also provide a measure for syncytia formation.
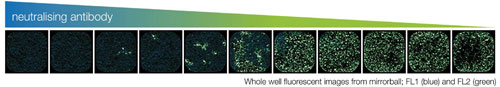
Figure 2. Whole-well fluorescence images from mirrorball; FL-1 (Hoechst, blue) and FL-2 (motavizumab AF-488, green) images
Discussion
This application note describes a simple and robust RSV neutralization assay. The unique optics of TTP Labtech’s mirrorball fluorescence cytometer enable a homogeneous assay format, thus removing the requirement for wash steps to remove unbound fluorescent detection reagent. This is particularly advantageous in the context of viral infectivity, where infection itself can promote the detachment of cells from the microplate and therefore lead to variable data in washed assay formats.
Not only do homogeneous assays reduce screening times by eliminating the requirement for wash and incubation steps, but they also minimize biosafety handling concerns, as the cells and virus are contained within the lidded (or sealed) microplate at all times.4
Mirrorball is the first system in its class to offer simultaneous scanning with multiple lasers, allowing direct correlation of fluorescence across lasers. We showed that normalization of the RSV infection signal [total intensity green (FL-2)] to the cell number eliminates data variability associated changes in cell number. The in situ read on the microplate preserves the cells in their culture environment, allowing changes in cell morphology to be measured.
For example, by determining the median area of nuclei in the well a measure of syncytia formation was obtained. The open source whole-well Tiff images generated by mirrorball readout present an avenue for further imaged-based analysis, if desired.

Figure 3 A. Concentration-response curve (total FL-2 intensity) B. Concentration-response curve (total FL-2 intensity/total FL-1 intensity) C. Concentration-response curve (median area of nuclei)
Mirrorball’s Key Benefits
- Simple, safe, and robust approach for high-throughput screening
- No-wash protocol
- Cells and virus remain safely contained within the assay plate compatible with high-density assay plates
- Whole-well scanning delivers robust data for uneven cell distribution
- Assay readout normalized to total cell count
- mirrorball’s in situ image-based read preserves cell morphology
References
1. McNamara & Smyth: “The pathogenesis of respiratory syncytial virus disease in childhood” British Medical
Bulletin (2002) 61 13–28
2. Gomez et al.: “Respiratory Syncytial Virus: pathology, therapeutic drugs and prophylaxis” Immunol. Lett. (2014) 162 237–47
3. Domachowske & Rosenberg: “Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment” Clin Microbiol Rev (1999) 12: 298–309
4. Rasmussen et al.: “Adapting high-throughput screening methods and assays for biocontainment laboratories” Assay Drug Dev Technol (2015) 13 44–54
Anne Hammerstein ([email protected]) is field application
scientist at TTP Labtech.







