By James Ross, PhD
Almost two years into the COVID-19 pandemic, the Food and Drug Administration (FDA) has approved very few therapeutics to treat this disease. With global cases on the rise and vaccination efforts facing ongoing supply and demand challenges, the world faces an urgent need to produce therapeutics that reduce the severity of COVID-19.
Many researchers believe the key to treating COVID-19 is to inhibit SARS-CoV-2 replication, since coronaviruses replicate via such a distinctive mechanism. To synthesize new copies of themselves, members of this virus family need to reorganize and recruit host cell membranes to form replication organelles.
Researchers have previously found promise in compounds that target the pathways that lead to the formation of these organelles. Now, researchers including Christopher F. Basler, PhD, and his colleagues at Georgia State University are testing whether inhibiting these pathways slows SARS-CoV-2 replication. Specifically, the Basler team is studying the ability of several small molecules to inhibit SARS-CoV-2’s cell-killing activity using the Maestro Z platform from Axion BioSystems.
Impedance-based bioelectronic assay
The Maestro Z uses electrical impedance monitoring to enable real-time, label-free monitoring of cell death across time periods ranging from seconds to weeks. The platform uses a 96-well plate with electrodes embedded in the bottom of each well. Small electrical currents are delivered through the electrodes at the base of the well, and the Maestro records the degree to which the contents of the well impede the signal.
Axion’s Impedance Module for the Maestro was designed to measure the dynamics of cell health: As cells attach to the well, impedance rises; as cells die, impedance falls. The health and integrity of the cells are reflected in the measured impedance. The team at Georgia State used this technique to monitor how effectively various compounds prevented cell death in response to SARS-CoV-2 infection.
The noninvasive nature of the impedance measurement means the Maestro Z is not susceptible to the limitations of typical live-cell imaging techniques. Live-cell imaging often requires dyes or probes that can alter cell viability and behavior. For example, these labels could create background noise, reducing sensitivity, or interfere with signaling pathways to directly disrupt the interactions being tested. Bioelectrodes, in contrast, measure the impact of SARS-CoV-2 on cell death without disturbing the natural behavior of the virus or the cells.
Furthermore, imaging data is often subject to the interpretation of the individual user, whereas bioelectronic assays offer an objective measure of cell viability. Finally, the 96-well format of the CytoView-Z plate enables the rapid analysis and direct comparison of multiple manipulations at once. This increased throughput lets Basler and scientists like him screen multiple drug candidates and study their activity in real time.
Validating the bioelectronic assay for screening COVID-19 antivirals
Basler and his colleagues used the Maestro Z (Figure 1) to monitor the effectiveness of various compounds in preventing cell death in response to SARS-CoV-2 infection. They first standardized their bioelectronic assay by measuring the behavior of SARS-CoV-2 in healthy cells.
After coating CytoView-Z 96-well electrode plates with fibronectin and filling each well with medium, the Basler team docked the plate in the Maestro Z instrument to measure baseline impedance. Then the team plated Vero E6 cells at a concentration of about 75,000 cells per well and left them for one hour to ensure even coverage of the well.
Plates were returned to the Maestro Z and incubated for 24 hours at body temperature (37°C) with 5% carbon dioxide. The team monitored the resistance, a component of impedance, as the Vero E6 cell monolayer formed. Finally, the team added SARS-CoV-2 virus to various wells at multiplicities of infection (MOIs) ranging from 0.0001 to 10 and measured impedance every minute for 72 hours. The team used the AxIS Z software to monitor continuous impedance data for each treatment group across the plate in real time.
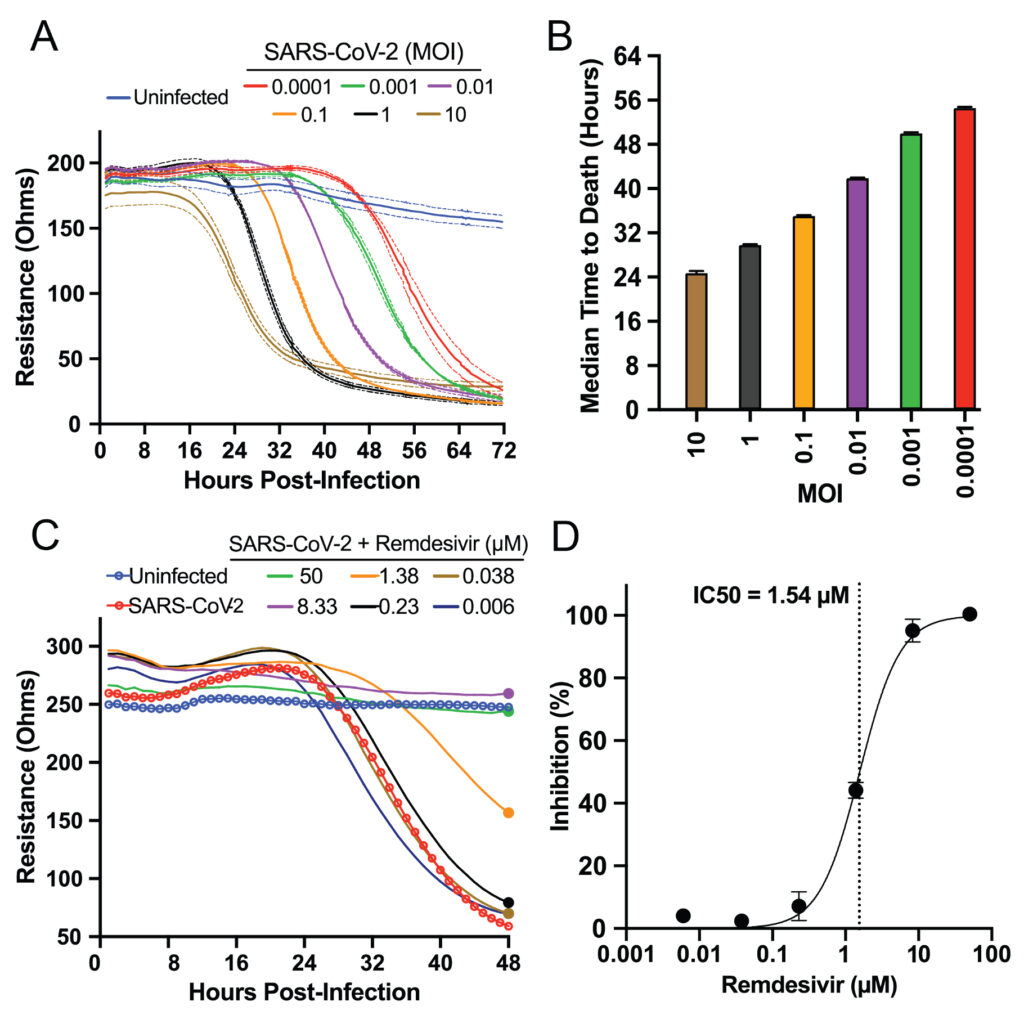
At the highest MOIs of 10 and 1, the Basler team detected a decrease in impedance—indicating cell death—as early as 18 to 24 hours post infection (Figure 2, A & B). At the lowest MOI, 0.0001, the team detected decreased impedance as late as 56 hours post infection, meaning the Maestro Z could detect the virus’s impact days later. All impedance levels reached their minimum between 32 and 72 hours post infection in an MOI-dependent manner.
As a positive control, Basler and colleagues measured the virus’s cytotoxic behavior on the Maestro Z in the presence of remdesivir, a known SARS-CoV-2 inhibitor (Figure 2, C & D). Using the AxIS Z software, they normalized raw resistance values for each well to the value at 1 hour post infection. Then, they calculated percent inhibition of each compound and dose by comparing each group to the untreated control group. They found the drug’s inhibitor concentration agreed with previous studies that used genetic methods to measure inhibition.
Overall, Basler’s team demonstrated that the Maestro Z could dynamically record the MOI-dependent cell-killing behavior of SARS-CoV-2, as well as the ability of remdesivir to inhibit this cytotoxicity. This work established the platform as an effective tool for screening potential SARS-CoV-2 therapeutics.
Testing the effects of novel compounds on SARS-CoV-2 kinetics
Moving on to their main objective, Basler and colleagues aimed to characterize inhibitors of SARS-CoV-2 replication. They first examined the effect of VPS34 inhibitors on SARS-CoV-2-mediated cell death. They chose this route because inhibiting VPS34, a kinase involved in cell membrane biology, had been found to impair infection of hepatitis C virus, tombusvirus, and coronavirus. The researchers also examined whether inhibiting fatty acid metabolism—another central pathway needed for SARS-CoV-2 replication—could prevent infection.
The researchers tested 10 concentrations of two well-known VPS34 inhibitors, VPS34-IN1 and PIK-III, and they measured impedance every minute for 72 hours. At the highest concentrations, these two compounds caused rapid cell death 1–20 hours post infection. At lower concentrations, however, these compounds were not toxic to the cells. This helped the team gather clues about the compounds’ potential toxicity in patients. After 48 hours, the team found that both compounds inhibited the virus’s cytopathic effects in a dose-dependent manner.
The team also tested two compounds that inhibit fatty acid metabolism, orlistat and triacsin C. Both compounds have demonstrated antiviral activity in the past. Testing the effect of these compounds using the same method as described above, the team determined that neither compound induced rapid cytotoxic effects like those seen with the VPS34 inhibitors. Both compounds inhibited SARS-CoV-2 cytopathic effects at higher concentrations, although complete inhibition was not achieved with orlistat. Together, these data demonstrated that VPS34 kinase activity and fatty acid metabolism play an important role in SARS-CoV-2 replication.
Other uses for the Maestro Z
The Basler team’s research exemplifies how the Maestro Z platform may serve as a new tool for COVID-19 therapeutic discovery and contribute to the fight against SARS-CoV-2. The platform also has applications that reach beyond antiviral discovery. It can be used for viral fitness comparison, neutralizing antibody detection, and viral titer determination.
Researchers have also used the Maestro Z platform to identify other entities that prevent or promote cell death, such as chimeric antigen receptor (CAR) T cells. Altogether, the platform expands
scientists’ ability to study interactions between cells, viruses, and
therapeutics in real time without interference. Maestro Z is generating new insights on therapeutic safety and efficacy that can contribute to faster therapeutic development.
James Ross, PhD, is the co-founder and chief technology officer of Axion Biosystems.





Comments are closed.