By Nisreen Shumayrikh, PhD
Therapeutic oligonucleotides are powerful and versatile biopolymers developed as drugs for clinical applications due to their straightforward synthesis and design. On the other hand, many of their most promising applications require therapeutic oligonucleotides to be stable in complex biological environments such as blood and living cells. The lifetime of administered oligonucleotides can be compromised significantly in vivo due to unintended interactions, for example, with enzymes that specifically degrade bacterial or viral DNA or RNA.1
To increase biostability, researchers have developed a variety of chemically-modified nucleotide analogs and include these in therapeutic oligonucleotides. However, these chemical modifications often profoundly impact hybridization properties and can lead to increased toxicity and immunogenicity in vivo.2,3
Scientists have begun to uncover a novel strategy to harness mirror-image isomers of nucleic acids (L-oligonucleotides) with the same physical and chemical properties as their biologically native D-counterparts.4 The only difference between L- and D-oligos is the arrangement of their atoms in space, making them a mirror image of each other: “enantiomers.” This difference can offer many advantages in L-oligos’ production and administration. First, they are resistant to nuclease degradation unlike traditional D-oligonucleotides. Second, they aren’t synthesized by biological systems, so their production requires chemical and easily scalable synthesis.
The most successful application of L-oligonucleotides to date has been the discovery and development of aptamers comprised entirely of L-DNA and/or L-RNA that are capable of recognizing, with high specificity and affinity, a broad range of ligands.5 These L-nucleic acid polymers are referred to as Spiegelmers (the word “Spiegel” means mirror in German).
To produce Spiegelmers, scientists use a technology that combines SELEX (Systematic Evolution of Ligands by EXponential Enrichment) with chemical mirroring technology that can be used against a wide variety of target molecules. SELEX is used for screening a library of up to 1015 single-stranded oligonucleotides that each fold into a distinct shape and may therefore fit well to a target of interest.
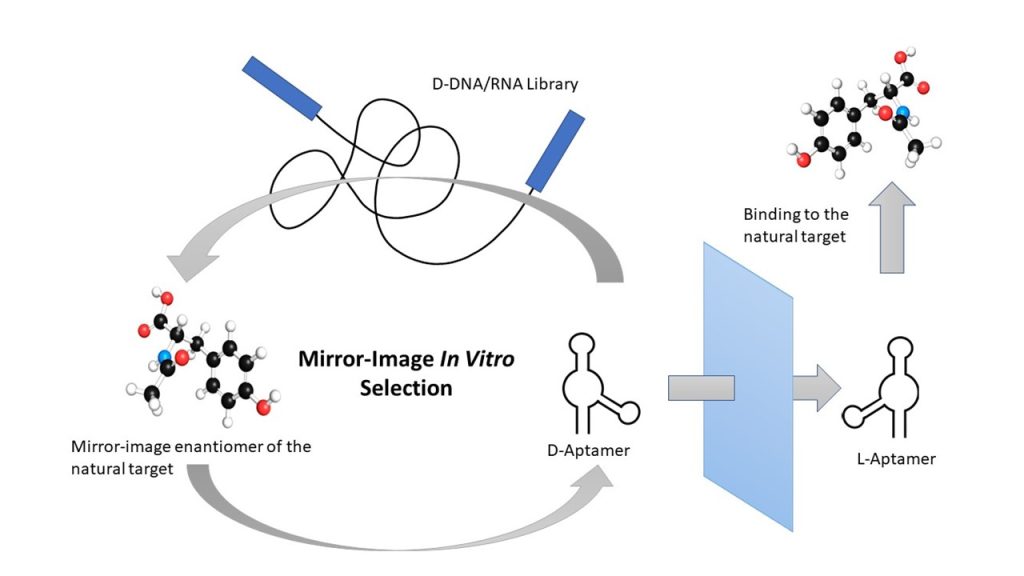
As shown in Figure 1, the in vitro section process is generally performed using the non-natural (mirror-image) L-enantiomer of the target ligand for which aptamers are initially selected using a traditional D-oligos library. This is referred to as “mirror-image” in vitro selection or “selection–reflection.” As a result, the enzymatic amplification of the D-oligos is maintained during the in vitro selection process. Once a suitable D-aptamer is identified, the corresponding L-aptamer (Spiegelmer) can be chemically synthesized and used to test its binding affinity to the desired natural target. Based on chirality rules, the Spiegelmer must bind to the native target with the same affinity as the originally selected D-aptamer against the mirror-image selection target.
To date, Spiegelmers have evolved to bind various targets, including small molecules, peptides, and proteins.6–7 Furthermore, Spiegelmers have been shown to retain all their highly desirable properties including their high affinity for targets while being nontoxic and nonimmunogenic in vivo.8 Indeed, several Spiegelmer therapeutic drugs have completed Phase I or II clinical trials. NOXXON Pharma (now named TME Pharma), a clinical-stage company based in Germany, has developed a novel therapy for treating the most aggressive cancers using their Spiegelmers platform. The team of scientists was able to identify NOX-A12 (olaptesed pegol), an L-enantiomer RNA that targets the chemokine stromal cell-derived factor 1 (SDF-1 or CXCL12), an essential signaling protein whose main role is leukocyte recruitment at inflammatory sites.9

Axel Vater, PhD, founder and CSO at Aptarion Biotech and formerly vp of drug discovery and preclinical research at NOXXON Pharma, said “NOX-A12 unites several mechanisms of action in one molecule: NOX-A12 can detach its target from negatively charged cell surfaces, and once the target is in solution, it is bound, neutralized, transported away from its original site and most likely co-eliminated with the drug through the liver.”
Vater’s team demonstrated the significance of this mechanism. Earlier studies have shown cancer cells synthesized excessive levels of the chemotactic molecule SDF-1 (CXCL12) to confuse and evade immune cells. NOX-12 removes excess SDF-1, particularly near blood vessels and, to a lesser extent, deeper inside the tumor. This forms an SDF-1 concentration gradient that the immune cells can follow, allowing them to attack the tumor.10
Vater said, “NOX-12 is currently being tested in hard-to-treat glioblastoma (brain cancer) with promising interim results in combination with an anti-PD1 antibody which blocks a second mechanism of immune evasion by tumors: a cell surface protein that tells immune cells to become inactive. Furthermore, its combination with the anti-angiogenic antibody bevacizumab has just reported positive interim results in hard-to-treat glioblastoma.”11
Vater explained that chemical synthesis of mirror-image enantiomers of the natural target is required to select Spiegelmers. “While peptide targets like chemokines, neuropeptides, and peptide hormones can be synthesized with ease from amino acids in the mirror-image D-configuration, larger protein targets are not amenable to this process,” said Vater. “Also, organic molecules need to be chirally inverted completely for this technology. This has been successfully done for some targets with one (adenosine, arginine) or even two (sphingosine-1-phosphate) chiral centers, but it becomes increasingly complex the more chiral centers there are in the molecule.”
By using chirally inverted enzymes in the SELEX process, it is possible to directly select Spiegelmers against targets in their natural enantiomeric form, said Vater. SELEX allows the direct amplification of the binding sequence from an oligonucleotide library in the L-configuration. 12,13
Both TME Pharma, which is focused on its clinical assets, and Aptarion Biotech, which developed its own immune-modulating C5alpha-neutralizing aptamer AON-D21 and continues to run the selection reflection process of the drug discovery engine for promising targets, are set to leverage the Spiegelmer technology and have shown that therapeutic L-aptamers have sufficient stability to function as potent and specific binders to key pharmacological targets in oncology but also in inflammatory diseases.
Nisreen Shumayrikh, PhD, is a freelance science writer.
References
- Lundin KE, Gissberg O, Smith CIE. Oligonucleotide Therapies: The Past and the Present. Hum Gene Ther. 2015;26(8):475-485. doi:10.1089/hum.2015.070
- Khvorova A, Watts JK. The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol. 2017;35(3):238-248. doi:10.1038/nbt.3765
- Shen W, De Hoyos CL, Sun H, Vickers TA, Liang XH, Crooke ST. Acute hepatotoxicity of 2’ fluoro-modified 5-10-5 gapmer phosphorothioate oligonucleotides in mice correlates with intracellular protein binding and the loss of DBHS proteins. Nucleic Acids Res. 2018;46(5):2204-2217. doi:10.1093/nar/gky060
- Klußmann S, Nolte A, Bald R, Erdmann VA, Fürste JP. Mirror-image RNA that binds D-adenosine. Nat Biotechnol. 1996;14(9):1112-1115. doi:10.1038/nbt0996-1112
- Young BE, Kundu N, Sczepanski JT. Mirror-Image Oligonucleotides: History and Emerging Applications. Chem – Eur J. 2019;25(34):7981-7990. doi:10.1002/chem.201900149
- Purschke WG, Eulberg D, Buchner K, Vonhoff S, Klussmann S. An L-RNA-based aquaretic agent that inhibits vasopressin in vivo. Proc Natl Acad Sci. 2006;103(13):5173-5178. doi:10.1073/pnas.0509663103
- Vater A, Klussmann S. Turning mirror-image oligonucleotides into drugs: the evolution of Spiegelmer(®) therapeutics. Drug Discov Today. 2015;20(1):147-155. doi:10.1016/j.drudis.2014.09.004
- Kulkarni O, Eulberg D, Selve N, et al. Anti-Ccl2 Spiegelmer permits 75% dose reduction of cyclophosphamide to control diffuse proliferative lupus nephritis and pneumonitis in MRL-Fas(lpr) mice. J Pharmacol Exp Ther. 2009;328(2):371-377. doi:10.1124/jpet.108.142711
- NOX-A12. Accessed November 9, 2022. https://www.tmepharma.com/index.php?option=com_content&view=article&id=21&Itemid=478
- Zboralski D, Hoehlig K, Eulberg D, Frömming A, Vater A. Increasing Tumor-Infiltrating T Cells through Inhibition of CXCL12 with NOX-A12 Synergizes with PD-1 Blockade. Cancer Immunol Res. 2017;5(11):950-956. doi:10.1158/2326-6066.CIR-16-0303
- Press Release Archive NOX-A12. Accessed November 12, 2022. https://www.tmepharma.com/index.php?option=com_content&view=article&id=51&Itemid=570
- Xu Y, Zhu TF. Mirror-image T7 transcription of chirally inverted ribosomal and functional RNAs. Science. 2022;378(6618):405-412. doi:10.1126/science.abm0646
- Pech A, Achenbach J, Jahnz M, et al. A thermostable d-polymerase for mirror-image PCR. Nucleic Acids Res. 2017;45(7):3997-4005. doi:10.1093/nar/gkx079







