GNAO1 (G Protein Subunit Alpha O1) is a protein-coding gene. Most patients with a GNAO1 neurodevelopmental disorder are diagnosed as infants or young children and the first GNAO1 patients were only identified in 2013. Scientists are still uncovering the spectrum of symptoms and impacts from mutations in GNAO1. Now, a new study by scientists from the University of Geneva (UNIGE) has found that a mutation in GNAO1 leads to the replacement of one amino acid by another in the protein sequence. They also found that a zinc molecule may partially restore the functioning of the protein affected by these mutations.
Their findings are published in the journal Science Advances in a paper titled, “Restoration of the GTPase activity and cellular interactions of Gαo mutants by Zn2+ in GNAO1 encephalopathy models.”
“De novo point mutations in GNAO1, a gene encoding the major neuronal G protein Gαo, have recently emerged in patients with pediatric encephalopathy having motor, developmental, and epileptic dysfunctions,” wrote the researchers. “Half of clinical cases affect codons Gly203, Arg209, or Glu246; we show that these mutations accelerate GTP uptake and inactivate GTP hydrolysis through displacement Gln205 critical for GTP hydrolysis, resulting in constitutive GTP binding by Gαo. However, the mutants fail to adopt the activated conformation and display aberrant interactions with signaling partners. Through high-throughput screening of approved drugs, we identify zinc pyrithione and Zn2+ as agents restoring active conformation, GTPase activity, and cellular interactions of the encephalopathy mutants, with negligible effects on wild-type Gαo.”
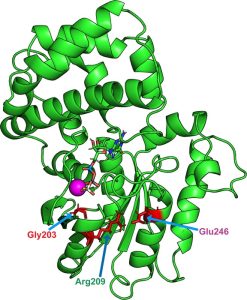
Mutations in the GNAO1 gene lead to the replacement of one amino acid in Gαo by another. These mutated proteins are not able to perform hydrolysis and are trapped in a permanent state of activation.
“These mutations are found to indirectly affect a crucial amino acid for GTP hydrolysis: glutamine 205. Normally, this glutamine is structurally located opposite GTP, which allows hydrolysis. However, this glutamine is displaced in the event of a pathologic mutation: this structural distance prevents this mechanism to take place,” noted Katanaev.
These mutations modify the ability of neurons to communicate with their environment.
“Ultimately, our goal is to try to find a treatment that could limit the symptoms of the disease and improve the quality of life of patients and their families,” added Katanaev.
The researchers performed high-throughput screening of thousands of approved drugs with the idea of identifying a molecule capable of reactivating hydrolysis. They discovered zinc pyrithione corrects the loss of intracellular interactions by bringing glutamine 205 close to its normal structural location, allowing GTP hydrolysis to occur.
‘‘This is an old antifungal and antibacterial drug used in cream form for certain skin diseases. We took the analysis a step further to see if all or part of this molecule was effective. It turns out that it is the zinc ion that is effective here. Very easy to find in any pharmacy, it is already approved for the treatment of mild depression, insomnia, and even in some developmental disorders in children,” said Katanaev.
The researchers confirmed their findings in a Drosophila fly model. ‘‘We modified the genome of flies to replicate the mutation of the GNAO1 gene, retaining a normal copy of the gene as in humans,” explained Mikhail Savitskiy, a researcher in Katanaev’s laboratory and a specialist in the modeling of diseases in Drosophila. “The flies had mobility issues and a reduced lifespan.”
This research provides insights into the molecular etiology of GNAO1 encephalopathy and defines a potential therapy for the patients.



