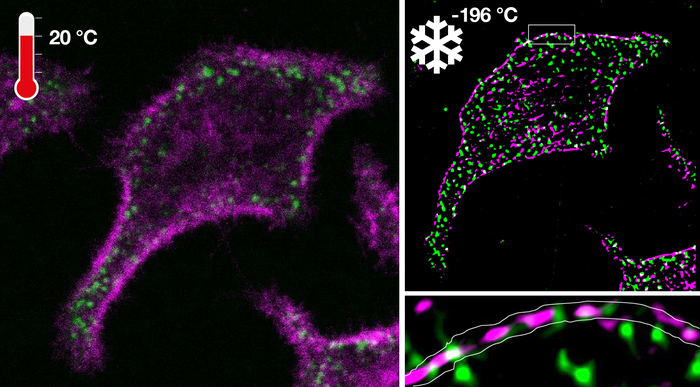
How well a structure or molecule is resolved by fluorescence microscopy depends on the amount of light that can be collected from the structure. In fluorescence microscopy, exposure time can be prolonged to increase the amount of detected light. However, microscopic structures exhibit random as well as directed motion. Prolonging the exposure time thereby leads to blurring of the structures.
Now, researchers have developed a technology to arrest molecular activity patterns during observation of their dynamics in living cells at any timepoint of interest—within milliseconds—directly on the fluorescence microscope. By doing this, both fundamental problems of motional blur and photodestruction can be bypassed at the same time.
This work is published in Science Advances in the article, “Ultrarapid cryo-arrest of living cells on a microscope enables multiscale imaging of out-of-equilibrium molecular patterns.”
The arrest is done by extremely fast cooling to temperatures (-196°C) where the molecular movement is virtually stopped. The arrest had to be very fast for two reasons. First, the energized microscopic patterns that define living cells disintegrate into the dead state if the arrest is too slow. Second, the speed of the arrest had to be faster than the process of ice formation, which would destroy the cells.
Ice formation happens extremely fast in the critical range between 0°C and -136°C. However, non-intuitively, at very low temperatures (below -136°C) ice crystals cannot form because the motion of water molecules is virtually stopped. This means that cooling had to be faster than 100,000°C per second.
The researchers have mastered this technical challenge by developing a ultrarapid cooling device that is integrated with a microscope where the cold of liquid nitrogen (-196°C) is accelerated under high pressure onto a diamond. The same diamond also holds the sample containing the cells on its opposing side. The high-pressure burst in combination with exceptional heat conductance of the diamond allowed the necessary high cooling rates to arrest cells at -196°C in their native configuration. This not only solved the problem of motional blur but also stopped photochemical destruction. This opens up the possibility of virtually infinite exposure, highlighting molecular patterns that are otherwise obscured in the noise.
Ultrarapid cryo-arrest allowed the use of normally destructive high laser powers to analyze native molecular patterns at tens-of-nanometer resolutions that were otherwise invisible. Because of the absence of photodestruction at -196°C, the same arrested cells could be observed by different microscopy modalities to measure patterns from the molecular to the cellular scale. This new technology led to the discovery of nanoscopic co-organization of an oncoprotein and a tumor suppressor protein that safeguards cells from exhibiting malignant behavior.
“This is an enabling step for fluorescence microscopy, especially the combination of super-resolution microscopy and microspectroscopy that allow the mapping of molecular reactions in cells at multiple scales,” said Philippe Bastiaens, PhD, professor and director of the
department for systemic cell biology, Max Plank Institute of Molecular Physiology, in Dortmund, Germany. “It will change the way we observe molecular organization and reaction patterns in cells and therefore provide more insight in the self-organizing capabilities of living matter.”