For optimal growth, tumors depend on the non-essential amino acids serine and glycine. Serine and glycine are biosynthetically linked and together, they provide the essential precursors for the synthesis of proteins, lipids, and nucleic acids that are critical to cancer cell growth, as well as supporting tumor homeostasis.
Over the last decade, scientists have learned that suppressing the amino acids from animal diets slows the growth of some tumors. Most researchers have focused on how these diets affect epigenetics, DNA metabolism, and antioxidant activity. However, researchers from the University of California (UC), San Diego, and the Salk Institute for Biological Studies have taken a different approach to slowing tumor growth, by focusing on serine’s metabolism.
Their study, “Serine restriction alters sphingolipid diversity to constrain tumor growth,” is published in Nature and led by Christian M. Metallo, PhD, professor of bioengineering at the Jacobs School of Engineering at UC San Diego.
“Serine, glycine, and other nonessential amino acids are critical for tumor progression, and strategies to limit their availability are emerging as potential therapies for cancer. However, the molecular mechanisms driving this response remain unclear and the effects on lipid metabolism are relatively unexplored,” the researchers wrote.
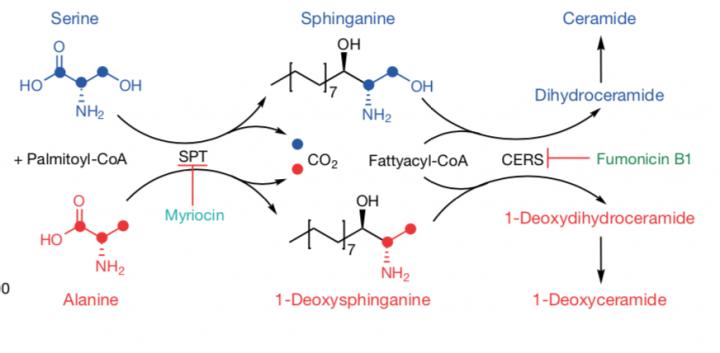
The enzyme serine palmitoyl-transferase (SPT) uses serine to make fatty molecules called sphingolipids, essential for cell function. If serine levels are low, the enzyme can go rogue and use a different amino acid such as alanine which results in the production of toxic deoxysphingolipids.
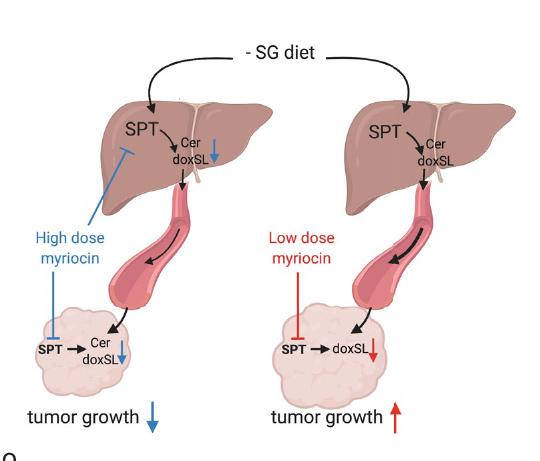
The researchers decided to exploit amino acid metabolism and the promiscuity of SPT to modulate the endogenous synthesis of toxic deoxysphingolipids and slow tumor progression. By restricting the dietary amino acids serine and glycine, which in turn targeted the serine synthesis enzyme phosphoglycerate dehydrogenase, the team induced tumor cells to produce a toxic lipid that slows cancer progression in mice.
Metallo noted that by linking serine restriction to sphingolipid metabolism, their findings may enable scientists to better identify which patients’ tumors are most sensitive to serine-targeted therapies.
The researchers fed xenograft model mice a diet low on serine and glycine. They then observed that SPT turned to alanine—an amino acid that is used in the biosynthesis of proteins—to produce toxic deoxysphingolipids instead of normal sphingolipids. The researchers were also able to use the amino-acid based antibiotic myriocin to inhibit SPT and deoxysphingolipid synthesis in mice fed low serine and glycine diets and found that tumor growth was improved.
Depriving an organism of serine for long periods of time leads to neuropathy and eye disease, Metallo pointed out. In a previous study, he and his team identified reduced levels of serine and accumulation of deoxysphingolipids as a key driver of a rare macular disease. However, serine restriction or drug treatments for tumor therapy would not require the prolonged treatments that induce neuropathy in animals or age-related diseases.
Further studies are needed to determine how this approach will translate to patients. However, the researchers believe their findings highlight the complexity of metabolism. “Our work highlights the beautiful complexity of metabolism as well as the importance of understanding physiology across diverse biochemical pathways when considering such metabolic therapies,” noted Metallo.