Researchers at New York University Pain Research Center have identified a mechanism that underlies inflammation and pain in the colon, and demonstrated that in mice with colitis, blocking a key receptor from entering colon cells can inhibit inflammation and pain. Their study identified PAR2 in endosomes, as a potential therapeutic target for inflammatory and painful diseases of the colon.
The team, led by senior study author Nigel Bunnett, PhD, professor and chair of the department of molecular pathobiology at NYU College of Dentistry, reported on the study in Proceedings of the National Academy of Sciences (PNAS), in a paper titled “Mice expressing fluorescent PAR2 reveal that endocytosis mediates colonic inflammation and pain.” In their report, they concluded, “PAR2 endocytosis and endosomal signaling disrupts the normal protective function of colonocytes and underlies colonic inflammation and pain. PAR2 in endosomes is a potential therapeutic target for inflammatory and painful diseases of the colon.”
The digestive tract is home to protease enzymes that break down proteins. These proteases come from a variety of sources, including the microbiome, inflammatory cells, or digestive enzymes in the intestines. While proteases are important for digestion and help to degrade proteins in the gut, many also signal cells by activating specific receptors. When proteases activate one such receptor—protease-activated receptor-2, or PAR2—on nerve cells, it produces pain. PAR2 is one of a major family of receptors known as G protein-coupled receptors (GPCRs), which regulate many processes in the body and are the targets of a significant number of clinically used drugs. “GPCRs are the largest family of transmembrane receptors, control most physiological and pathological processes, and are the target of >30% of approved drugs,” the authors wrote. However, they acknowledged, “Despite their medical importance, the effects of disease on the function and subcellular distribution of GPCRs are poorly understood.”
Studies have shown that proteases and PAR2 are involved in gastrointestinal diseases and pain. But scientists haven’t had much understanding of the receptor’s signaling mechanism and how it induces pain. “Proteases and PAR2 have been implicated in colonic diseases, including inflammatory bowel disease (IBD), irritable bowel syndrome, and cancer,” the team continued. “However, the signaling mechanisms by which PAR2 induces disease are incompletely understood.”
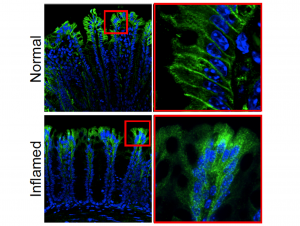
First, to pinpoint the location of PAR2 in the gut, the researchers created a mouse model in which the gene for PAR2 was fused to a green fluorescent protein. When a cell expressed PAR2, it lit up green, allowing the researchers to precisely locate where the receptor was positioned. Using this system the investigators found that PAR2 was very highly expressed in cells lining the small and large intestines, as well as the colon’s nerve fibers.
Further investigations then showed a key difference in the location and behavior of PAR2 in healthy mice versus animals with colitis. In healthy mice, PAR2 was found on the surface of colon cells, but in mice with colitis, it shifted from the surface of cells to compartments within cells called endosomes. When the receptor moved into endosomes, it generated signals that caused inflammation and pain by disrupting the normal protective function of cells lining the colon. “We detected a major redistribution of PAR2 from the basolateral membrane to early endosomes of colonocytes in two preclinical models of IBD, which is likely due to increased proteolytic activity in the inflamed colon,” the investigators stated. “Endocytosis allows the assembly of a PAR2, Gα, and β-arrestin signaling complex that mediates sustained increases in colonocyte paracellular permeability and persistent inflammation and hyperalgesia of the colon.”
So, if PAR2 moving from the surface of cells into endosomes leads to inflammation and pain, could blocking the receptor from entering cells limit inflammation and pain? To test this idea, the researchers prevented the movement of PAR2 into cells by knocking down the expression of a protein called dynamin-2. Through this approach, they showed that keeping the receptor out of cells did, in fact, inhibit signaling and significantly reduced pain and inflammation. “Knockdown of dynamin-2 (Dnm2), the major colonocyte isoform, and Dnm inhibition attenuated PAR2 endocytosis, signaling complex assembly and colonic inflammation and hyperalgesia,” the scientists stated.
The combined findings suggest that PAR2—and specifically PAR2 in endosomes—may be a useful target in treating pain in inflammatory bowel disease. “This could be achieved through blocking PAR2 from entering cells, as we did in this study by inhibiting dynamin-2,” said Bunnett. “It could also mean getting drugs that activate PAR2 not just to the surface of cells, but into the interior of cells using nanoparticles to reach the receptor in endosomes.”
The authors further concluded, “Therapeutic targeting of PAR2 in endosomes, which might be achieved using lipidated antagonists or endosomally targeted nanoparticles, could restrict endosomal signaling and ameliorate IBD and other inflammatory and painful diseases associated with enhanced proteolysis and PAR2 activation.”






