During implantation, the human embryo is hidden inside a “black box.” Although peeking inside has been prohibited by the 14-day rule, which restricts in vitro studies to preimplantation events, a new experimental model, one that incorporates embryonic stem cells, has been used to observe processes that ordinarily occur in embryos only after the first 14 days of development. By revealing these events, which include the emergence of the human body plan, the new model could help scientists deliver boon after boon: infertility treatments, new approaches to preventing miscarriages and birth defects, platforms for generating transplant tissues, and treatments for many diseases.
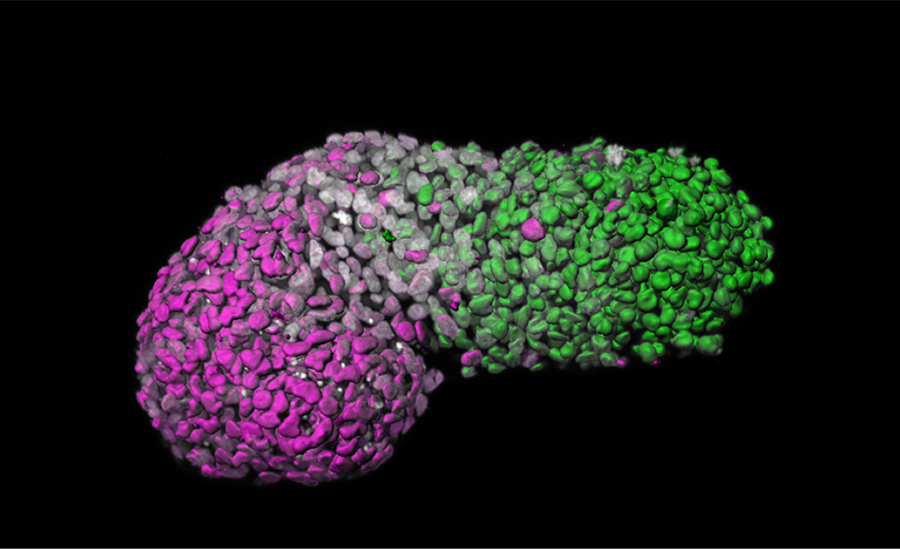
The new model is a human gastruloid, a three-dimensional assembly of stem cells that was developed by scientists from the University of Cambridge and the Hubrecht Institute in the Netherlands. These scientists reported that their gastruloid can capture certain aspects of gastrulation, the process by which an embryo transitions from a rounded shape to an elongated shape. During gastrulation, three distinct layers of cells are formed in the embryo that will later give rise to all the body’s major systems: the ectoderm layer will give rise to the nervous system; the mesoderm layer, the muscles; and the endoderm layer, the gut.
The scientists described their human gastruloid in a new study (“An in vitro model for early anteroposterior organization during human development”) that appeared in Nature. “Our model produces part of the blueprint of a human,” said Alfonso Martinez-Arias, PhD, professor at the University of Cambridge’s department of genetics, who led the study. “It’s exciting to witness the developmental processes that until now have been hidden from view—and from study.”
To make gastruloids in the lab, the scientists placed defined numbers of human embryonic stem cells into small wells, where the stem cells formed tight aggregates. After treatment with chemical signals, the gastruloids were seen to lengthen along a head to tail axis, known as the anteroposterior axis, turning on genes in specific patterns along this axis.
The scientists noted that although model organisms have provided much insight into this gastrulation, very little about the process in humans, “owing to the difficulty of obtaining embryos at such early stages of development and the ethical and technical restrictions that limit the feasibility of observing gastrulation ex vivo.”
According to Martinez-Arias and Naomi Moris, PhD, junior research fellow at the University of Cambridge who participated in the study, human gastruloid models should be considered ethical. “Human gastruloids do not show any evidence of cell types associated with the brain, nor do they form the cells required for implantation,” they indicated. “Significantly, they lack the morphology (shape) of an early human embryo, and therefore do not manifest human organismal form. As such, they are non-intact, non-autonomous, and non-equivalent to human embryos, and do not have human organismal potential.”
Martinez-Arias and Moris also indicated that gastruloids are distinct from embryoid bodies (EBs), which have been used for many years to promote the differentiation of pluripotent stem cells toward multiple lineages. “The different cell types that emerge in EBs remain disorganized and often fail to represent the organization of the embryo,” they noted. “One potential reason for these differences is that EBs are generated from thousands of cells and under different culture conditions, while gastruloids are generated from a few hundred cells under specific conditions that favor the spatial and temporal patterning of the group of cells.”
By looking at which genes were expressed in these human gastruloids at 72 hours of development, the Cambridge University and Hubrecht Institute researchers found a clear signature of the event that gives rise to important body structures such as thoracic muscles, bone, and cartilage—but no structures containing brain cells.
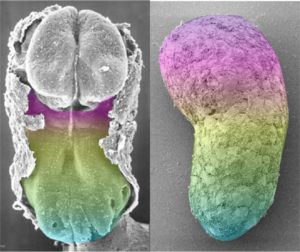
“In the human gastruloids, the pattern of gene expression—with a central somitic domain and posterior presomitic domain of a similar length—leads us to suggests that 72-h human gastruloids might serve as a model for some of the features of late CS8 or early CS9 human development,” the authors of the Nature article wrote. That is, the researchers judged the equivalent human embryonic age of the gastruloids by comparing them to the Carnegie Collection of Embryology. This official collection contains a continuum of human embryos, including day-by-day growth over the first eight weeks. They suggest that gastruloids partially resemble 18–21-day-old human embryos.
“With the current protocol, the majority of human gastruloids curl or retract after 72 h; this probably represents a technical limitation, and extension beyond this point will require further study,” the article’s authors added. “The lack of anterior neural and extra-embryonic lineages that is characteristic of gastruloids raises important questions about the self-organization of the mammalian body plan, but also removes several of the ethical considerations that are associated with prolonged culturing of human embryo.”
Model organisms including mice and zebrafish have previously enabled scientists to gain some insights into human gastrulation. However, these models may behave differently from human embryos when the cells start to differentiate. Animal models can respond differently to certain drugs: the anti-morning sickness drug thalidomide, for example, passed clinical trials after testing in mice but subsequently led to severe birth defects in humans.
“[The new gastruloid system] will allow us to reveal and probe the processes of early human embryonic development in the lab for the first time,” said Moris, the first author of the current study. “Our system is a first step toward modeling the emergence of the human body plan, and it could prove useful for studying what happens when things go wrong, such as in birth defects.”