A live imaging study has identified epigenetic differences between the brains of people with schizophrenia and those who don’t have the neuropsychiatric disorder. Researchers at the Center for Biomedical Imaging at Massachusetts General Hospital (MGH) carried out positron emission tomography (PET) imaging using a radiotracer called [11C]Martinostat to examine the expression of the epigenetic histone deacetylase (HDAC) enzyme HDAC2 in regions of the brain known to be important for cognitive functioning. The results indicated that HDAC expression was reduced in key areas of the brains of people with schizophrenia (SCZ) or schizoaffective disorder.
While reduced HDAC levels have previously been reported in postmortem brains from people with schizophrenia, use of the imaging agent Martinostat has for the first time allowed measurement of HDAC expression in living people. “HDAC enzymes can profoundly influence gene expression in the brain and may alter cognition,” said Tonya Gilbert, Ph.D., who was previously at the Martinos Center. “With Martinostat, instead of being confined to analyzing small tissue samples, as in earlier studies, we can now assay HDAC expression and distribution across the entire living brain in a single PET scan. Beyond comparisons between healthy and disease states, this will enable long-term studies exploring the relationship between HDAC levels and disease onset, progression, and symptom severity.” Dr. Gilbert is lead author of the team’s published paper in The Journal of Clinical Investigation (JCI), which is titled, “PET neuroimaging reveal histone deacetylase dysregulation in schizophrenia.”
Although a link between epigenetic mechanisms and cognitive function has been implicated through previous preclinical and postmortem human studies, to date no direct link has been made in living human brains because there haven’t been any suitable epigenetic imaging tools, the authors explain. The emergence of neuroimaging tools that can measure the distribution of epigenetic enzymes is now giving scientists new ways of exploring how the amount and location of enzymes is linked with brain anatomy and function and disease phenotypes.
Previous preclinical and human postmortem research has linked HDACs to cognitive circuitry. “Of the epigenetic enzymes that can regulate gene transcription and influence behavior, HDACs have emerged as potential targets for therapeutic interventions,” the researchers wrote. Studies have also shown that HDAC inhibitors can have beneficial effects in mouse models of severe neuropsychiatric and neurodegenerative disorders. Such research collectively suggested that “HDAC-related mechanisms may play a fundamental role in human cognition,” the team noted.
Their previous studies had also found reduced HDAC2 mRNA levels in the dorsolateral prefrontal cortex (DLPFC) region of the post-mortem brains of donors with schizophrenia, but not other psychiatric disorders evaluated. The DLPFC is a key region of the brain that coordinates human cognition, including executive functions such as working memory, planning, and mental flexibility, which are impaired in patients with schizophrenia. “Based on these data we hypothesized that regional [11C]Martinostat uptake would be lower in the DLPFC of patients with SCZ,” they reasoned.
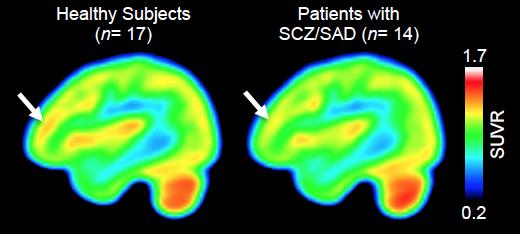
Martinostat has been developed by a Marinos Center team headed by Jacob Hooker, Ph.D., associate professor of radiology at Harvard Medical School and senior author of the latest JCI paper. The MGH researchers have previously used Martinostat and PET to map HDAC expression in the brains of healthy individuals. For the studies reported in JCI, the team compared HDAC expression in the DLPFC region of the brain in 14 individuals with either schizophrenia or schizoaffective disorder, and 17 control participants. As expected, the Martinostat PET scans showed that uptake of the tracer—indicating binding to HDAC2—was much lower in the DLFPC of participants with schizophrenia, than it was in the DLFPC regions of the brain among control participants. The degree of reduced uptake also correlated with worse cognitive performance scores.
Interestingly, whole brain scans indicated that while HDAC expression was also lower in some areas adjacent to the DLFPC, it was, unexpectedly, higher in cerebral white matter and in the cerebellum, which has also been linked with schizophrenia. “These data may suggest that abnormal HDAC expression, in either direction, could have negative effects on cognition,” the authors stated.
“While we found preliminary correlations between the relative Martinostat signal in the cerebral white matter and multiple aspects of cognitive testing—such as processing speed and working memory—we don’t yet know which types of cognitive factors HDAC is most closely correlated with,” said Dr. Gilbert, who is now with Eikonizo Therapeutics, but continues to collaborate with Dr. Hooker’s team. “Moving forward we hope to apply advanced cognitive measures to our studies to help better understand the relationship between HDAC levels and human cognition.”
“In conclusion, our study presents in vivo evidence of human neuroepigenetic dysregulation in SCZ and provides the foundation for using [11C]Martinostat PET to study the role of HDACs in human cognition,” the authors stated. “We’re very interested in understanding the role of HDACs across mental illness, as well as neurodevelopmental and neurodegenerative disease, added Dr. Hooker. “Martinostat is being used by several teams at MGH to study Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis, substance use disorder, and several non-brain but HDAC-relevant diseases like heart failure and cancer. It’s also being used at other academic medical centers to help learn how epigenetic alterations relate to disease processes.”