Uncovering how the SARS-CoV-2 virus enters into cells is one of the key pieces of the puzzle to blocking its infection. It has been shown previously that SARS-CoV-2 uses the ACE2 receptor to infect human cells. Now, new research from two separate manuscripts shows that to efficiently infect human cells, SARS-CoV-2, the virus that causes COVID-19, is able to use a receptor called neuropilin-1, which is very abundant in many human tissues including the respiratory tract, blood vessels, and neurons.
The work is published in Science, in two papers published back to back. One paper is titled, “Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity,” while the second paper is titled, “Neuropilin-1 is a host factor for SARS-CoV-2 infection.”
Unlike other coronaviruses, which cause common colds and mild respiratory symptoms, SARS-CoV-2, is highly infective and transmissive. Until now, major questions have remained unanswered as to why SARS-CoV-2 readily infects organs outside of the respiratory system, such as the brain and heart.
“That SARS-CoV-2 uses the receptor ACE2 to infect our cells was known, but viruses often use multiple factors to maximize their infectious potential,” said Giuseppe Balistreri, PhD, head of the viral cell biology research group at the University of Helsinki involved in the first study.
To infect humans, SARS-CoV-2 must first attach to the surface of human cells that line the respiratory or intestinal tracts. Once attached, the virus invades the cell then replicates multiple copies of itself. The replicated viruses are then released leading to the transmission of SARS-CoV-2.
Unlike other respiratory viruses, SARS-CoV-2 also infects the upper respiratory system including the nasal mucosa, and consequently spreads rapidly. “This virus is able to leave our body even when we simply breathe or talk,” Balistreri added. “The starting point of our study was the question why SARS-CoV, a coronavirus that led to a much smaller outbreak in 2003, and SARS-CoV-2, spread in such a different way even if they use the same main receptor ACE2,” explained Ravi Ojha, a researcher in the Balistreri lab and one of the main contributors of the study.
To understand how these differences can be explained, the researchers took a look at the viral surface proteins, the spikes, that, like hooks, anchor the virus to the cells. Balistreri reveals that “when the sequence of the SARS-CoV-2 genome became available, at the end of January, something surprised us. Compared to its older relative, the new coronavirus had acquired an ‘extra piece’ on its surface proteins, which is also found in the spikes of many devastating human viruses, including Ebola, HIV, and highly pathogenic strains of avian influenza, among others. We thought this could lead us to the answer.”
Together, the coordinated team of international researchers, including more than 30 scientists from Germany, Finland, Estonia, and Australia, looked at whether neuropilins were important for infection by SARS-CoV-2.
They found that neuropilin-1, known to bind furin-cleaved substrates, “significantly potentiates SARS-CoV-2 infectivity, an effect blocked by a monoclonal blocking antibody against NRP1.” Their experiments showed that “a SARS-CoV-2 mutant with an altered furin cleavage site did not depend on neuropilin-1 for infectivity.” In addition, pathological analysis of human COVID-19 autopsies revealed SARS-CoV-2 infected cells including olfactory neuronal cells facing the nasal cavity positive for neuropilin-1.
The second study, another huge collaboration, used multiple approaches to discover that SARS-CoV-2 recognizes a protein called neuropilin-1 on the surface of human cells to facilitate viral infection.
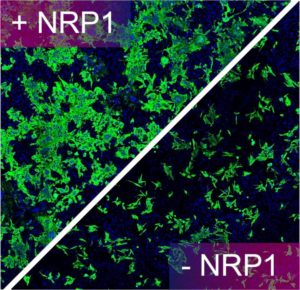
More specifically, the team showed that the host protease furin cleaves the full-length precursor S glycoprotein into two associated polypeptides: S1 and S2. The authors write that, “Cleavage of S generates a polybasic Arg-Arg-Ala-Arg C-terminal sequence on S1, which conforms to a C-end rule (CendR) motif that binds to cell surface neuropilin-1 (NRP1) and neuropilin-2 (NRP2) receptors.” The group used X-ray crystallography and biochemical approaches to show that the S1 CendR motif directly bound NRP1. Blocking this interaction using RNAi or selective inhibitors reduced SARS-CoV-2 entry and infectivity in cell culture.
“Once we had established that the Spike protein bound to neuropilin-1 we were able to show that the interaction serves to enhance SARS-CoV-2 invasion of human cells grown in cell culture. Importantly, by using monoclonal antibodies—lab-created proteins that resemble naturally occurring antibodies—or a selective drug that blocks the interaction we have been able to reduce SARS-CoV-2’s ability to infect human cells. This serves to highlight the potential therapeutic value of our discovery in the fight against COVID-19.”
New antiviral strategy in the making
By specifically blocking neuropilin-1 with antibodies, the researchers were able to significantly reduce infection in laboratory cell cultures. “If you think of ACE2 as a door lock to enter the cell, then neuropilin-1 could be a factor that directs the virus to the door. ACE2 is expressed at very low levels in most cells. Thus, it is not easy for the virus to find doors to enter. Other factors such as neuropilin-1 might help the virus finding its door,” said Balistreri.
Balistreri cautiously concluded: “it is currently too early to speculate whether blocking directly neuropilin could be a viable therapeutic approach, as this could lead to side effects. This will have to be looked at in future studies. Currently, our laboratory is testing the effect of new molecules that we have specifically designed to interrupt the connection between the virus and neuropilin. Preliminary results are very promising and we hope to obtain validations in vivo in the near future.”



