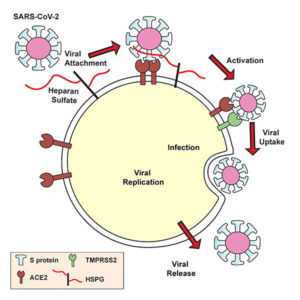
Lung cells put out a welcome mat for SARS-CoV-2, a welcome mat that has been equated with ACE2, a receptor molecule. But ACE2 may account for just half the mat, a new study indicates. The other half may consist of heparan sulfate. In fact, SARS-CoV-2 appears to set its spike protein down on heparan sulfate first, whereupon the spike protein’s receptor-binding domain adopts an open conformation, the better to engage ACE2.
To keep SARS-CoV-2 from crossing the threshold, that is, from being taken into the host cell, scientists have been looking for ways to interfere with the spike–ACE2 interaction. Going forward, scientists may have more luck sending SARS-CoV-2 on its way if they remove heparan sulfate or prevent it from appearing in the first place. Essentially, scientists could take away a crucial part of the welcome mat. Alternatively, scientists could preoccupy SARS-CoV-2 with heparin, a form of heparan sulfate that is already a widely used medication to prevent and treat blood clots.
These possibilities were suggested by the scientists who discovered how cellular heparan sulfate facilitates the spike–ACE2 interaction. The scientists conducted a study at the University of California, San Diego (UCSD), School of Medicine to uncover evidence suggesting that heparan sulfate acts as a coreceptor priming the spike for ACE2 interaction.
“ACE2,” said Jeffrey Esko, PhD, distinguished professor of cellular and molecular medicine at UCSD, “isn’t the whole picture.” Esko led the study with visiting scholar Thomas Mandel Clausen, PhD, and postdoctoral researcher Daniel Sandoval, PhD.
These scientists and their colleagues presented their findings September 14 in Cell, in an article titled, “SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2.” The article describes how the authors, using human cells cultured in the laboratory, demonstrated that infection rates could be reduced approximately 80–90% by manipulating heparan sulfate or introducing exogenous heparin: “Unfractionated heparin, non-anticoagulant heparin, heparin lyases, and lung heparan sulfate potently block spike protein binding and/or infection by pseudotyped virus and authentic SARS-CoV-2 virus.”
The article also describes how the scientists explored heparan sulfate’s role in the pathogenic mechanism of SARS-CoV-2 infection: “Docking studies suggest a heparin/heparan sulfate-binding site adjacent to the ACE2 binding site. Both ACE2 and heparin can bind independently to spike protein in vitro and a ternary complex can be generated using heparin as a scaffold. Electron micrographs of spike protein suggest that heparin enhances the open conformation of the RBD that binds ACE2.”
Late one Friday afternoon in March 2020, Clausen was tired and, he admits, putting off his experiments. Instead, he perused the latest research coming out about SARS-CoV-2. That’s when he came across a preliminary study that suggested an interaction between the coronavirus’s spike protein and another carbohydrate related to heparan sulfate.
“I ran down to Daniel to tell him to look at the study—and of course, he was already thinking the same thing,” recalled Clausen, who is also an associate professor at the University of Copenhagen in Denmark.
Within a week, the team was testing their theories in the lab. They discovered that the SARS-CoV-2 spike protein binds to heparin. The team also drilled down to uncover the exact part of the SARS-CoV-2 spike protein that interacts with heparin—the receptor-binding domain. When heparin is bound, the receptor-binding domain opens up and increases binding to ACE2. The virus, they found, must bind both heparan sulfate on the cell surface and ACE2 to get inside human lung cells grown in a laboratory dish.
With this viral entry mechanism established, the researchers next set about trying to disrupt it. They found that enzymes that remove heparan sulfate from cell surfaces prevent SARS-CoV-2 from entering cells. Likewise, treatment with heparin also blocked infection. The heparin treatment worked as an antiviral at doses currently given to patients, even when the researchers removed the anticoagulant region of the protein—the part responsible for preventing blood clots.
The findings are still far from translating into a COVID-19 treatment for people, advised Esko. Researchers will need to test heparin and heparan sulfate inhibitors in animal models of SARS-CoV-2 infection. In a related study, UCSD scientists are also exploring the role human microbiomes, including the bacteria that live in and on the body, play in altering heparan sulfate and thus influencing a person’s susceptibility to COVID-19.
“This is one of the most exciting periods of my career,” Esko declared. “All of the things we’ve learned about heparan sulfate and the resources we’ve developed over the years have come together with a variety of experts across multiple institutions who were quick to collaborate and share ideas. If there’s a silver lining to this pandemic, I hope it’s that the scientific community will continue to work rapidly together like this to address other problems.”







