The complex networks of fluid-filled tubes and loops that exist in human organs have three-dimensional structures whose connections vary, depending on the organ. How these connections and topology develop during the development of an embryo, remains unclear.
Now, using tissue reconstitution and quantitative microscopy, metrics for organ development have been defined for the first time. In a new study, an international team of researchers provides the necessary tools to transform the field of organoids into an engineering discipline to develop model systems for human development.
This research is published in Nature Physics, in the paper, “Topological morphogenesis of neuroepithelial organoids.”
While past studies have shown how cell mechanics induce local shape changes during the development of an organism, it is not clear how the connectivity of tissues emerges.
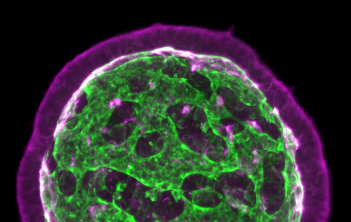
By combining imaging and theory, Keisuke Ishihara, PhD, a postdoctoral researcher in the Tanaka lab at the Research Institute of Molecular Pathology (IMP) in Vienna, Austria, sought to provide insight into this developmental process. Ishihara used organoids derived from mouse embryonic stem cells that form a complex network of epithelia, which line organs and function as a barrier.
“I still remember the exciting moment when I found that some organoids had transformed into tissues with multiple buds that looked like a bunch of grapes,” noted Keisuke. “Describing the change in the three-dimensional architecture during development proved to be challenging, though,” he added. “I found that this organoid system generates astonishing internal structures with many loops or passages, resembling a toy ball with holes.”
Studying the development of tissues in organoids has several advantages: they can be observed with advanced microscopy methods, making it possible to see dynamic changes deep inside the tissue. They can be generated in large numbers and the environment can be controlled to influence development.
The researchers were able to study the shape, number, and connectivity of the epithelium. They tracked the changes in the internal structure of organoids over time.
The authors wrote that “tissue topology and shape is governed by two distinct modes of topological transitions. One mode involves the fusion of two separate epithelia and the other involves the fusion of two ends of the same epithelium.”
Keisuke noted, “We discovered that tissue connectivity emerges from two different processes: either two separate epithelia fuse or a single epithelium self-fuses by fusing its two ends, and thereby creating a doughnut-shaped loop.”
They also identified a pharmacologically accessible pathway that “regulates the frequency of two modes of epithelial fusion and demonstrates the control of organoid topology and shape.”
The findings suggest, based on the theory of epithelial surfaces, that the inflexibility of epithelia is a key parameter that controls epithelial fusion and in turn the development of tissue connectivity.
“We hope that our findings will lead to a fresh view of complex tissue architectures and the interplay between shape and network connectivity in organ development,” the authors noted. “Our experimental and analysis framework will help the organoid community to characterize and engineer self-organizing tissues that mimic human organs. By revealing how cellular factors influence organ development, these results may also be useful for developmental cell biologists who are interested in organizational principles.”