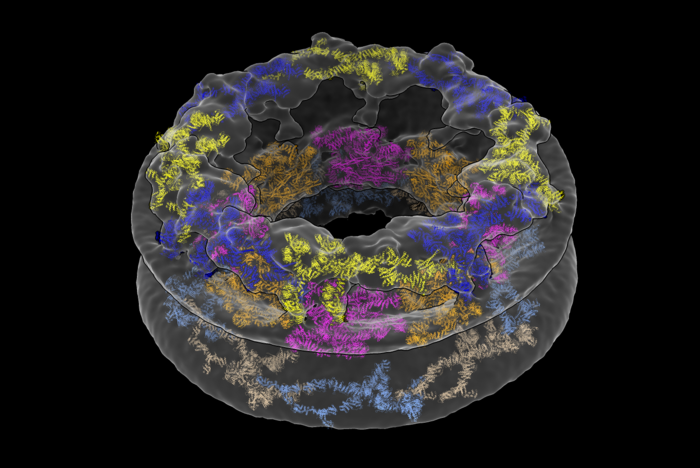
Nuclear pore complexes (NPCs) allow for the transport of cargo across the nuclear envelope between the nucleus and cytoplasm. They are composed of roughly thirty different nucleoporins that are distributed in three main substructures (the inner, cytoplasmic, and nucleoplasmic rings) around the central transport channel. Now, a new study uses cryo-electron tomography to generate a structural model for the human NPC directly inside cells. Their results reveal that the pore has larger dimensions than previously thought, emphasizing the importance of analyzing complex molecules in their native environments.
This work is published in Nature in the paper, “The cellular environment shapes the nuclear pore complex architecture.”
“We’ve shown that the cellular environment has a significant impact on large structures like the NPC, which was something we weren’t expecting when we started,” said Thomas Schwartz, PhD, professor of biology at MIT. “Scientists have generally thought that large molecules are stable enough to maintain their fundamental properties both inside and outside a cell, but our findings turn that assumption on its head.”
Traditionally, scientists have broken the NPC up into individual components to study it using a method called X-ray crystallography. The group from MIT, together with researchers from the University of Zurich, employed two cutting-edge approaches to solve the pore’s structure: cryo-focused ion beam (cryo-FIB) milling and cryo-electron tomography (cryo-ET). The researchers captured cross-sections of the cells that included NPCs, rather than simply looking at the NPCs in isolation.
“The amazing thing about this approach is that we’ve barely manipulated the cell at all,” Schwartz said. “We haven’t perturbed the cell’s internal structure. That’s the revolution.”
What the researchers saw was quite different from existing descriptions of the NPC. They were surprised to find that the innermost ring structure, which forms the pore’s central channel, is much wider than previously thought. When left in its natural environment, the pore opens up to 57 nm—resulting in a 75% increase in volume compared to previous estimates. The team was also able to take a closer look at how the NPC’s various components work together to define the pore’s dimensions and overall architecture.
“We’ve shown that the cellular environment impacts NPC structure, but now we have to figure out how and why,” said Anthony Schuller, PhD, a Helen Hay Whitney postdoctoral fellow in the Schwartz lab and first author on the paper. Not all proteins can be purified, he added, so the combination of cryo-ET and cryo-FIB will also be useful for examining a variety of other cellular components. “This dual approach unlocks everything.”
“The paper nicely illustrates how technical advances, in this case, cryo-electron tomography on cryo-focused ion beam milled human cells, provide a fresh picture of cellular structures,” said Wolfram Antonin, PhD, professor of biochemistry at RWTH Aachen University in Germany, who was not involved in the study. The fact that the diameter of the NPC’s central transport channel is larger than previously thought hints that the pore could have impressive structural flexibility. “That may be important for the cell to adapt to increased transport demands,” Antonin explained.
The team hopes to further understand how the size of the pore effects which molecules can pass through. For instance, scientists only recently determined that the pore was big enough to allow intact viruses like HIV into the nucleus. The same principle applies to medical treatments: only appropriately-sized drugs with specific properties will be able to access the cell’s DNA.
Schwartz is especially curious to know whether all NPCs are created equal, or if their structure differs between species or cell types. “We’ve always manipulated cells and taken the individual components out of their native context,” he said. “Now we know this method may have much bigger consequences than we thought.”