Oxidative stress can motivate cells to live according to the Nietzschean sentiment that goes, “What does not kill us makes us stronger.” Cells, after a brief oxidant challenge, may react by pumping up their production of antioxidants, developing an enhanced antioxidant capacity, and becoming more resistant to subsequent oxidant challenges. These cellular responses, which were recently investigated by Salk Institute scientists, suggest that short-term stress may lead to long-term adaptations that could keep cells healthy longer, staving off aging and disease.
During normal metabolism, a chemical byproduct called superoxide builds up within mitochondria. If enough superoxide accumulates, it can become toxic, prompting mitochondria to produce superoxide dismutase (SOD), an enzyme that converts superoxide to less toxic molecules. If too little SOD is available, however, mitochondria suffer stress—which isn’t entirely harmful. The stress can promote an adaptation called mitohormesis, promoting beneficial physiological responses and enhancing longevity. But how?
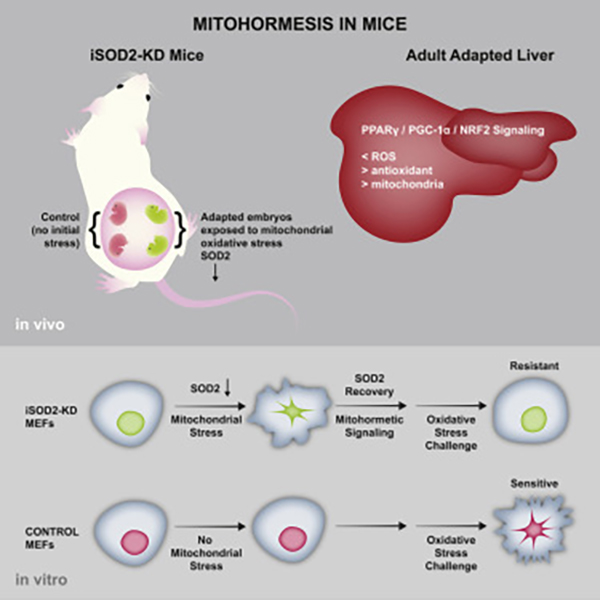
That’s the question a Salk Institute team led by Gerald Shadel, Ph.D., professor, molecular and cell biology laboratory, and Audrey Geisel Chair in biological sciences, sought to answer. To interrogate mitohormetic pathways in mammals, the team generated mice in which mitochondrial superoxide dismutase 2 (SOD2) can be knocked down in an inducible and reversible manner (iSOD2-KD mice).
Essentially, the Salk Institute scientists generated a model that let them turn off antioxidant production in mitochondria, but in a reversible way. “We were able to induce this stress for specific time windows and see how cells responded,” asserts Dr. Shadel.
Dr. Shadel and colleagues used their model to investigate how short-term cellular stress caused by mitochondrial superoxide very early in development might affect health later in life. Specifically, they studied the embryonic development of genetically identical mice, half of which had a molecular “off” switch for SOD. These mice were subjected to brief stress.
The results of this work appeared August 16 in the journal Cell Metabolism, in an article titled, “Mitohormesis in Mice via Sustained Basal Activation of Mitochondrial and Antioxidant Signaling.” It described how embryonic mitochondrial oxidant stress results in adaptive changes in adult liver. That is, the livers of adapted mice had increased mitochondrial biogenesis and antioxidant gene expression and fewer reactive oxygen species.
“Gene expression analysis implicated non-canonical activation of the Nrf2 antioxidant and PPARγ/PGC-1α mitochondrial signaling pathways in this response,” wrote the article’s authors. “Transient SOD2 knockdown in embryonic fibroblasts from iSOD2-KD mice also resulted in adaptive mitochondrial changes, enhanced antioxidant capacity, and resistance to a subsequent oxidant challenge.”
After the mice were born and continued to grow to adulthood, the two groups looked very similar. But liver samples taken when they were four weeks old told a strikingly different story: the mice whose SOD enzyme had been turned off briefly to trigger stress in mitochondria had—surprisingly—higher levels of antioxidants, more mitochondria and less superoxide buildup than the mice who had not experienced stress. Additionally, cells grown in dishes, half which contained the SOD switch, showed the same results: those that experienced brief periods of stress turned out to be stress resistant and healthier from a cellular perspective.
When the team analyzed which genes were being activated in both the lab dishes and the liver samples of all the mice, they found unexpected molecular pathways at work in the SOD group that were reprogramming mitochondria to produce fewer toxic molecules while simultaneously increasing the cells' antioxidant capacity.
“We propose,” the article’s authors wrote, “that mitohormesis in response to mitochondrial oxidative stress in mice involves sustained activation of mitochondrial and antioxidant signaling pathways to establish a heightened basal antioxidant state.”
“We are excited to test if the unique mitohormesis signaling pathways we will elucidate in this new mouse model can be targeted to prevent common age-related disease like cancer, Alzheimer's, and heart disease,” adds Dr. Shadel.