Scientists at Memorial Sloan Kettering Cancer Center, New York have discovered a critical mechanism that allows cancer cells to grow in an inflammatory environment. ENPP1, a highly accessible molecule on the surface of cancer cells, is a key player in this mechanism and can be leveraged to sensitize certain types of cancers to immunotherapeutic drugs, the study reports.
Free floating DNA snippets in the cytosol signal red alert in cancer cells with unstable chromosomes. This warning signal activates a specific innate immunity pathway called the cGAS-STING pathway that results in inflammation.
Despite the inflammatory immune response triggered by the cancer cells that could act as a self-destruct signal, it has long been observed that cancer cells not only survive but thrive in this highly unfavorable environment.
“The elephant in the room is that we didn’t really understand how cancer cells were able to survive and thrive in this inflammatory environment,” says Samuel Bakhoum, MD, PhD, physician-scientist at Memorial Sloan Kettering Cancer Center and a member of the Human Oncology and Pathogenesis Program.

In a new study titled “Metastasis and immune evasion from extracellular cGAMP hydrolysis“published in the journal Cancer Discovery, Bakhoum and his team report that part of the reason that allows cancer cells to thrive in inflammatory environments has to do with a molecule on cancer cell surfaces that destroys the warning signals before they can reach surrounding immune cells.
These findings help explain why certain tumors do not respond to immunotherapeutic drugs and suggest strategies that can lead to successful therapeutic interventions mediated by the sensitizing of cancer cells.
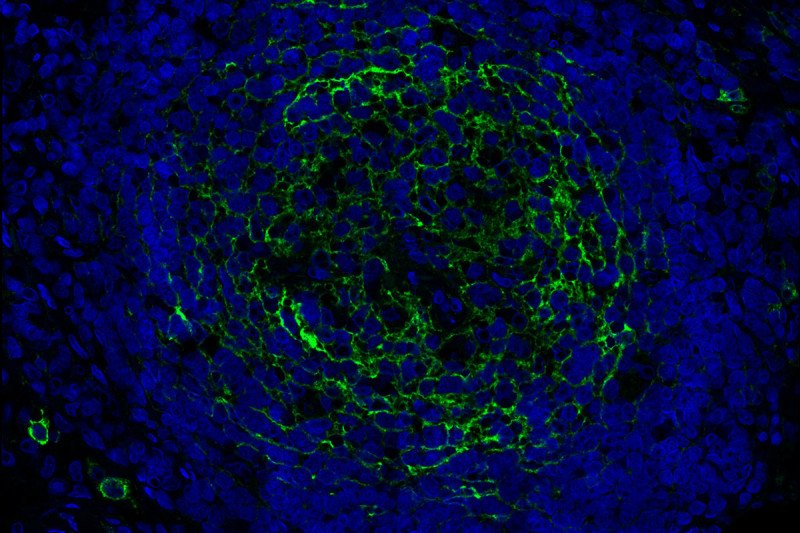
When DNA from an unstable cancer chromosome finds its way into a cell’s cytoplasm, cGAS binds to it, forming a compound molecule called cGAMP, which serves as a warning signal. Inside the cell, this warning signal activates an immune response called STING, which addresses the immediate problem of a potential threat.
Many cGAMP complexes also travel out of the cell and alert neighboring immune cells. This activates their STING pathway and unleashes an immune attack against the cancer cell with unstable chromosomes.
Bakhoum’s lab had earlier shown that cGAS-STING signaling in cancer cells causes them to adopt attributes of immune cells such as the ability to migrate, which promotes the spread of cancer to other locations, a process called metastasis. This provided part of the answer to the question of how cancer cells survive inflammation and spread in the process.
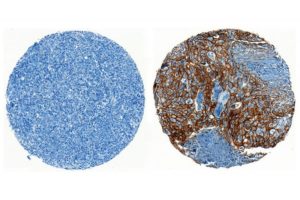
The current research shows how the cancer cells thwart warning signals that activated cGAS-STING complexes release into the environment. A scissor-like protein called ENPP1, that coats the outer surface of cancer cells, shreds the signals, providing a second way for cancer cells to suppress an innate immune response. When cGAMP finds its way outside the cell, ENPP1 digests it and prevents the signal from reaching neighboring immune cells simultaneously releasing an immune-suppressing molecule called adenosine, which also subdues the inflammation.
Bakhoum and his colleagues show turning on ENPP1 suppresses immune responses and increases metastasis while turning it off enables immune responses and reduces metastasis. Mouse models of breast, lung, and colorectal cancers were used as experimental models in the study to demonstrate ENPP1 acts like a control switch for immune suppression and metastasis. The presence of ENPP1 on samples of human cancers correlate with increase in metastasis and resistance to immunotherapy.
Several companies including Volastra Therapeutics that Bakhoum and his colleagues founded, are now developing drugs to inhibit ENPP1 on cancer cells. The location of ENPP1 in the surface of cancer cells and its specific expression, makes it an easy and effective drug target. “One of the things I would be really proud of is if this research ends up helping patients soon, given that we only just started this work in 2018,” says Bakhoum.”You’re simultaneously increasing cGAMP levels outside the cancer cells, which activates STING in neighboring immune cells, while you’re also preventing the production of the immune-suppressive adenosine. So, you’re hitting two birds with one stone.”