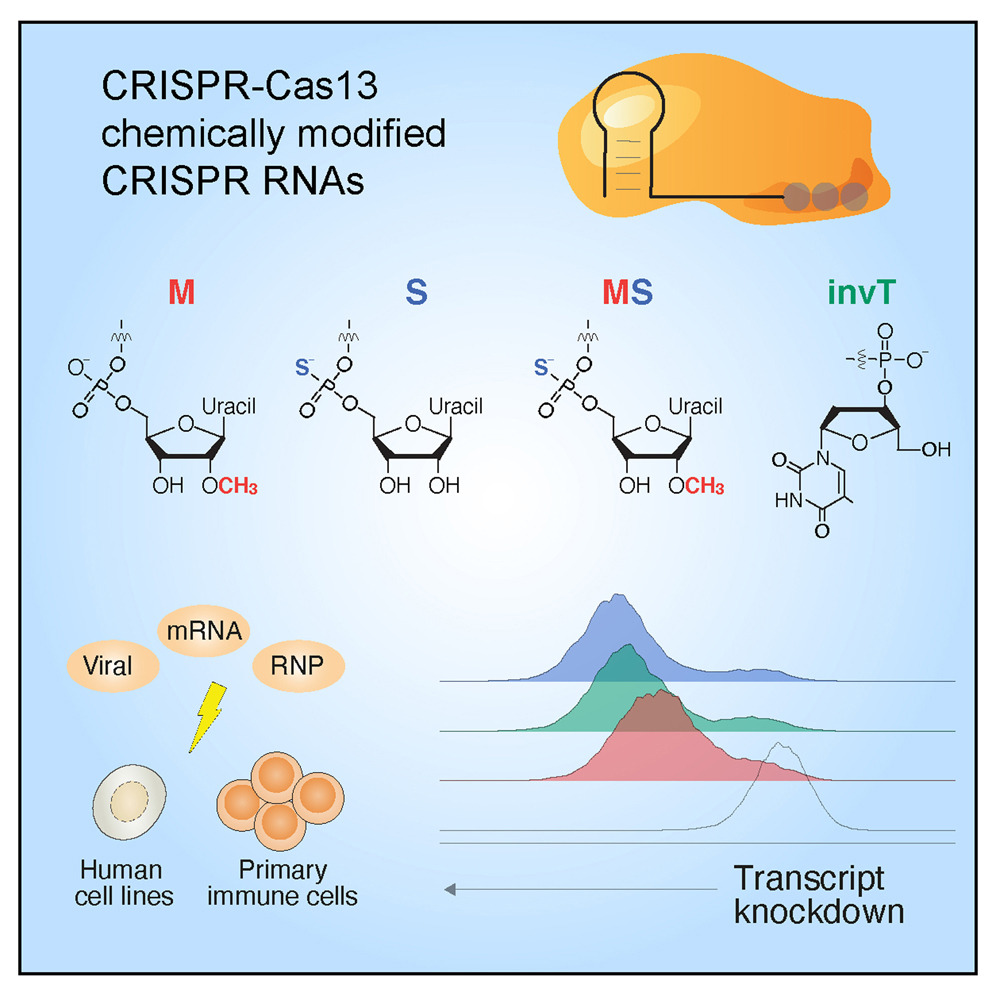
Typically, CRISPR-based genome editing is used to target and cut DNA. Recently, however, RNA-targeting CRISPR-Cas13 proteins have emerged as tools to alter gene expression. Now, researchers explore different chemical RNA modifications at various positions and detail how the modified guides increase efficiencies of CRISPR activity (from 2- to 5-fold) over unmodified guides. They also show that the optimized chemical modifications extend CRISPR-targeting activity from 48 hours to four days.
The research is published in Cell Chemical Biology in the paper, “Chemically modified guide RNAs enhance CRISPR-Cas13 knockdown in human cells.”
“CRISPR RNA guide delivery can be challenging, with knockdown time limited due to rapid guide degradation. We were inspired by the guide modifications developed for other DNA-targeting CRISPRs and wanted to test if chemically modified guides could improve knockdown time for RNA-targeting CRISPR-Cas13 in human cells,” said Alejandro Méndez-Mancilla, PhD, a postdoc at New York Genome Center (NYGC) and co-first author of the study.
Drawing on the lab’s previous study that outlined principles for optimal Cas13 guide design, published in Nature Biotechnology in March 2020, the researchers systematically applied and tested a variety of chemical modifications. For example, they found that adding three bases with a different type of chemical bond linking them to each other (phosphorothioate modification) extended RNA target knockdown ability by several days in a human cell line. In primary T cells, the phosphorothioate modification resulted in 60–65% knockdown of expression of CD46, a receptor involved in immune system regulation, as compared to achieving 40–45% knockdown when using an unmodified guide.
To apply these optimized chemical modifications, the research team, which included collaborators at Synthego and New England BioLabs, targeted cell surface receptors in human T cells from healthy donors and a “universal” segment of the genetic sequence shared by all known variants of the RNA virus SARS-COV-2.
The team found that certain methylation and inverted terminator modifications also improved Cas13 activity. For all modifications, the placement of these modified RNA bases was crucial. When placed incorrectly, the modifications resulted in guide RNAs that did not function.

“These modified guides further expand the toolbox for genome and transcriptome engineering. For non-coding elements in the human genome, targeting DNA may not be effective, and other organisms, such as RNA viruses like coronavirus or flu, cannot be targeted at all,” said Neville Sanjana, PhD, core faculty member at NYGC.
The team’s test to knockdown the universal leader sequence segment of the RNA virus SARS-CoV-2 in human cells, for example, is only possible using an RNA-targeting CRISPR like Cas13. SARS-CoV-2 enters the cells and releases its RNA genome, which is then transcribed into smaller RNAs, referred to as subgenomic RNAs. These subgenomic RNAs are responsible for making the different proteins required for the virus to replicate and then infect other cells. The universal leader sequence is found at the beginning of each subgenomic RNA. Thus, an effective approach for targeting the universal leader sequence may protect cells against further viral replication and infection.
In addition, CRISPR-Cas13’s ability to modulate genetic expression without permanently altering the underlying DNA genome sequence, as do DNA-targeting CRISPRs like Cas9 or Cas12a, is critical. “Transient modulation to spur genetic expression outcomes is often preferred in biomedical research and drug development. For example, the messenger RNA vaccines for SARS-CoV-2 express transiently but create an immune memory that lasts beyond their expression lifetime,” noted Sanjana.