Acetate is a precursor of acetyl-CoA, which is used by cells for the synthesis of fatty acids and cholesterol to build up the cell membrane. Now researchers at the RIKEN Center for Integrative Medical Sciences (IMS) report they have discovered that acetate could trigger an immune response against potentially harmful bacteria.
Their findings are published in the journal Nature in a paper titled, “Acetate differentially regulates IgA reactivity to commensal bacteria,” and may lead to the development of new ways to regulate the balance of intestinal bacteria.
“The balance between bacterial colonization and its containment in the intestine is indispensable for the symbiotic relationship between humans and their bacteria,” wrote the researchers.
“One component to maintain homeostasis at the mucosal surfaces is immunoglobulin A (IgA), the most abundant immunoglobulin in mammals. Several studies have revealed important characteristics of poly-reactive IgA, which is produced naturally without commensal bacteria. Considering the dynamic changes within the gut environment, however, it remains uncertain how the commensal-reactive IgA pool is shaped and how such IgA affects the microbial community.
“Here we show that acetate—one of the major gut microbial metabolites—not only increases the production of IgA in the colon, but also alters the capacity of the IgA pool to bind to specific microorganisms including Enterobacterales.”
IgA is the first line of defense in the resistance against infection, via inhibiting bacterial and viral adhesion to epithelial cells and by neutralization of bacterial toxins and viruses. Before this new finding, it was unknown what could trigger IgA responses to bacteria in a changing intestinal environment.
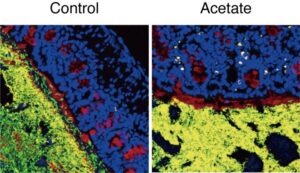
“SCFAs including acetate are easily absorbed in the stomach and proximal small intestine, so it was difficult to investigate the effects of orally administered SCFAs in the distal intestine such as the colon, where the intrinsic SCFA levels are high,” said Ohno. “Our collaborators have developed a method that can efficiently deliver the metabolites into the distal intestine, which enabled us to analyze the effects of SCFAs on the immune system there.” Other SCFAs, such as propionic acid or butyric acid did not affect IgA.
The researchers also found that the type of bacteria to which IgA binds depends on whether acetate is present or not.
“At first we thought that acetate simply increases IgA equally against all commensal bacteria,” explained Ohno. “It was rather surprising to see that it preferentially enhances production of IgA against certain microbes through collaboration with other immune cells.” In fact, the experiments showed that acetate only increased IgA production when potentially harmful bacteria were present.
This study revealed that acetate produced by bacteria can change the balance of IgA in the intestines. The researchers identified a role for gut microbial metabolites in the regulation of differential IgA production to maintain mucosal homeostasis.
“Accumulating evidence suggests the involvement of gut microbiota in many human diseases. IgA is one of the most efficient ways to control the microbiota, and therefore we think our findings are a basis for this regulatory mechanism. Since the functions of metabolites remain largely unknown, we will keep focusing on host-microbe interactions through these small molecules to reveal how they affect human pathophysiology,” said Ohno.