Recent modeling research suggests that malaria-related deaths in sub-Saharan Africa in 2020 could be more than double those of 2019 if malaria-prevention activities are interrupted due to COVID-19. The malaria burden is heavily concentrated in sub-Saharan Africa, where COVID-19 cases are also rising.
A new study, conducted by an international team, demonstrated that the parasites that cause malaria are heavily dependent on enzymes in red blood cells, protein kinases, where the parasites hide and proliferate. The study, “Analysis of erythrocyte signaling pathways during Plasmodium falciparum infection identifies targets for host-directed antimalarial intervention,” is published in Nature Communications.
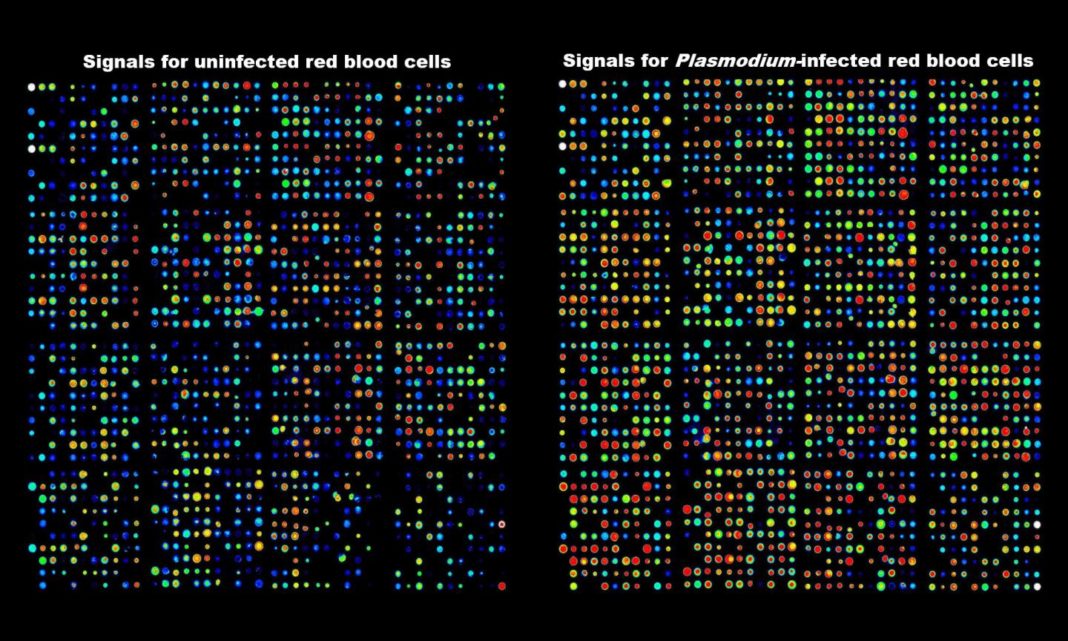
Intracellular pathogens, such as Plasmodium falciparum, mobilize host signaling pathways of their host cell to promote their own survival. According to the authors, emerging evidence suggests that signal transduction elements are activated in a-nucleated erythrocytes in response to infection with malaria parasites, but the extent of this phenomenon remains unknown.
In this study, the team of researchers “fill this knowledge gap through a comprehensive and dynamic assessment of host erythrocyte signaling during infection with Plasmodium falciparum.” Using arrays of 878 antibodies directed against human signaling proteins to interrogate the activation status of host erythrocyte phospho-signaling pathways at three blood stages of parasite asexual development, they reveal a dynamic modulation of many host signaling proteins across parasite development.
The authors focused on the hepatocyte growth factor receptor (c-MET) and the MAP kinase pathway component B-Raf, providing a proof of concept that human signaling kinases identified as activated by malaria infection represent attractive targets for antimalarial intervention.
The study supports further study of drugs developed for cancer, which inactivate the protein kinases and are highly effective in killing the parasite as an alternative to drugs that target the parasite itself. This strategy is promising as it suggests a promising path forward using repurposed drugs for malaria.

Lead author, Jack Adderley, PhD, a postdoctoral fellow at RMIT University in Melbourne, Australia, noted that the analysis revealed which of the host cell enzymes were activated during infection, revealing novel points of reliance of the parasite on its human host.
“This approach has the potential to considerably reduce the cost and accelerate the deployment of new and urgently needed antimalarials,” Adderley said. “These host enzymes are in many instances the same as those activated in cancer cells, so we can now jump on the back of existing cancer drug discovery and look to repurpose a drug that is already available or close to completion of the drug development process.”
As well as enabling the repurposing of drugs, the approach is likely to reduce the emergence of drug resistance, as the pathogen cannot escape by simply mutating the target of the drug, as is the case for most currently available antimalarials.
Christian Doerig, associate dean for the Biomedical Sciences Cluster at RMIT and senior author of the paper, said the findings were exciting, as drug resistance is one of the biggest challenges in modern healthcare, not only in the case of malaria, but with most infectious agents, including a large number of highly pathogenic bacterial species.
“We are at risk of returning to the pre-antibiotic era if we don’t solve this resistance problem, which constitutes a clear and present danger for global public health. We need innovative ways to address this issue,” he said.
“By targeting the host and not the pathogen itself, we remove the possibility for the pathogen to rapidly become resistant by mutating the target of the drug, as the target is made by the human host, not the pathogen.”
The distribution of long-lasting insecticidal mosquito nets has had a major role in the control of malaria in sub-Saharan Africa, and many countries have net-distribution campaigns planned for 2020. Other anti-malaria strategies are a welcome addition to net-based prevention strategies.