A few rotten apples may indeed spoil the whole bunch—if the bunch happens to be an organoid, a cluster of cells grown in the laboratory to mimic an organ. Kidney organoids, for example, are used to study how real kidneys work and respond to newly developed treatments. But if kidney organoids contain wayward non-kidney cells, they may fail to mimic real kidneys properly, leading scientists astray.
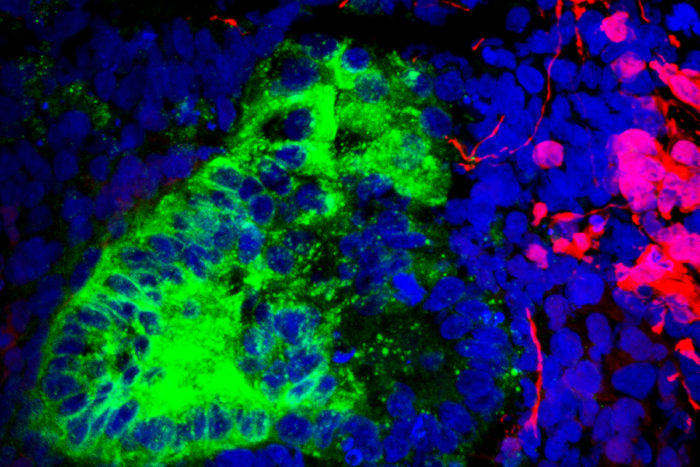
According to scientists based at Washington University School of Medicine (WUSM) in St. Louis, kidney organoids may end up accommodating brain and muscle cells. Such unwelcome guests make up only 10–20% of an organoid’s cells, the scientists found, but their presence indicates that the protocols used to coax stem cells into becoming kidney cells may inadvertently produce other cell types.
At first glance, the discovery might appear a setback for using kidney organoids as stand-ins for human kidneys, but kidney organoids, the scientists say, have ways to ensure they remain an exclusive destination. The scientists found an easy way to prevent most of those wayward cells from forming. The scientists also suggest that their approach could be adopted by other scientists who find rogue cells in other organoids, such as those of the brain, lung, or heart.
Details of the work appeared November 15 in the journal Cell Stem Cell, in an article titled, “Comparative Analysis and Refinement of Human PSC-Derived Kidney Organoid Differentiation with Single-Cell Transcriptomics.” Essentially, the article describes how gate crashers work, and how they can be excluded from organoid functions.
Reconstructing lineage relationships by pseudotemporal ordering identified ligands, receptors, and transcription factor networks associated with fate decisions,” the article indicated. Some of these fate decisions, the article continued, involved the development of brain cells: “Brain-derived neurotrophic factor (BDNF) and its cognate receptor NTRK2 were expressed in the neuronal lineage during organoid differentiation.”
Inhibiting the neuronal pathway, the scientists reported, improved organoid formation. Neuronal interlopers were reduced by 90% without affecting kidney differentiation. According to the scientists, this result highlights the power of single-cell technologies to characterize and improve organoid differentiation.
“There’s a lot of enthusiasm for growing organoids as models for diseases that affect people,” said the article’s senior author, Benjamin D. Humphreys, M.D., Ph.D., director of nephrology at WUSM. “But scientists haven’t fully appreciated that some of the cells that make up those organoids may not mimic what we would find in people. The good news is that with a simple intervention, we could block most of the rogue cells from growing.”
For the current study, the researchers looked at two recipes widely used by scientists worldwide to grow kidney organoids. One starts with embryonic stem cells approved for research by the National Institutes of Health (NIH), and the other begins with induced pluripotent stem cells, which are reprogrammed from adult cells and have the ability to develop into any type of human cell.
A cocktail of drugs and growth factors are added to the stem cells, channeling their development into kidney cells. After growing the organoids in the lab for four weeks, a time frame long enough for the cells to specialize, the researchers asked: What kinds of cells did we get?
Rather than conduct a spot check to identify cells that made up the organoids, the researchers relied on a relatively new technique to take a deep dive. Using single-cell RNA sequencing, they analyzed the activity of many thousands of genes in 83,130 cells from 65 kidney organoids.

“This generates massive amounts of data, and there’s no way our brains can make sense of it all,” Dr. Humphreys explained. “But computers can easily compare gene activity across 83,000 cells and, using artificial intelligence, group cell types together based on their gene expression. So, rather than looking for cells that we think we thought we’d find in the organoid, it helped us find cells even if we’d never imagined they’d be there.”
“Progress to develop better treatments for kidney disease is slow because we lack good models,” Dr. Humphreys explained. “We rely on mice and rats, and they are not little humans. There are many examples of drugs that have done magically well at slowing or curing kidney disease in rodents but failed in clinical trials. So, the notion of channeling human stem cells to organize into a kidney-like structure is tremendously exciting because many of us feel that this potentially eliminates that ‘lost in translation’ aspect of going from a mouse to a human.”