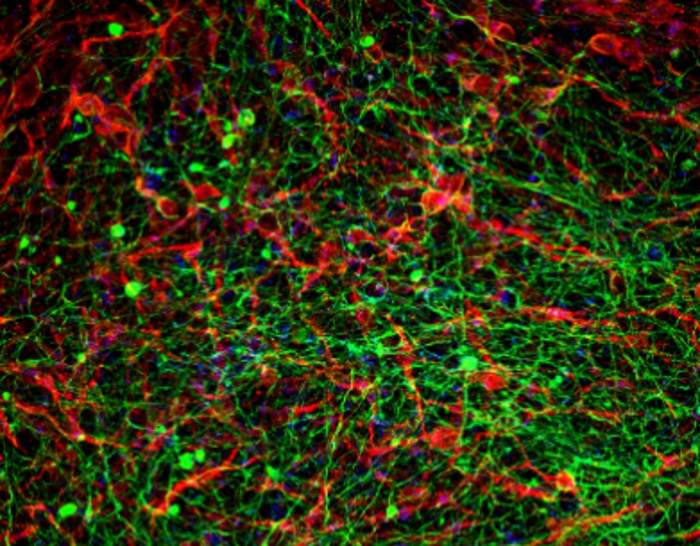
Scientists at the Sagol Center for Regenerative Biotechnology, the Shmunis School of Biomedicine and Cancer Research, and the department of biomedical engineering at Tel Aviv University have generated functioning 3D spinal cord implants, using human tissue samples, through a process that mimics the development of the spinal cord in human embryos. Applied in a lab model with long-term chronic paralysis, the implants successfully restored walking abilities in 80% of tests.
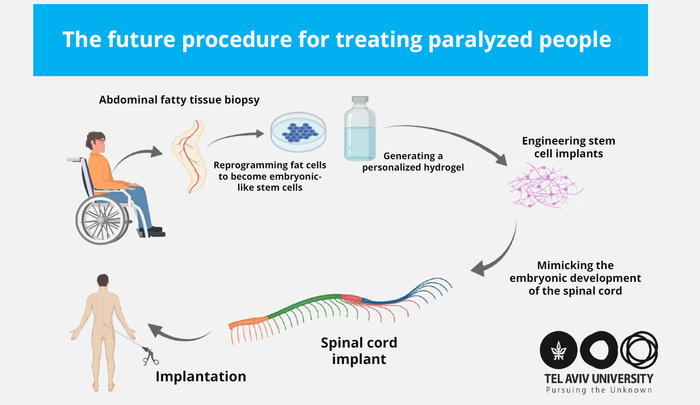
The researchers, led by a team in the laboratory of Tal Dvir, PhD, professor, Tel Aviv University, are now preparing for clinical trials in human patients. They hope that within a few years the engineered tissues will be implanted in paralyzed individuals enabling them to stand up and walk again.

Dvir said, “This is the first instance in the world in which implanted engineered human tissues have generated recovery in an animal model for long-term chronic paralysis—which is the most relevant model for paralysis treatments in humans. There are millions of people around the world who are paralyzed due to spinal injury, and there is still no effective treatment for their condition … We hope to reach the stage of clinical trials in humans within the next few years, and ultimately get these patients back on their feet.”
The researchers reported on their results in Advanced Science, in a paper titled, “Regenerating the injured spinal cord at the chronic phase by engineered iPSCs-derived 3D neuronal networks.”
Traumatic spinal cord injury (SCI) has an immediate and catastrophic impact on all aspects of the patient’s health and quality of life, the authors noted. The primary injury causes direct damage, which commonly results in the death of cells, as well as disruption to the blood-spinal cord barrier, and degradation of the extracellular matrix (ECM). “These processes initiate a secondary proinflammatory injury cascade, which leads to progressive tissue damage resulting in the formation of a glial scar,” the team continued. Healthy neural tissue around the injury site contains cues that could promote tissue repair, but the lack of what the researchers described as “a permissive microenvironment” for cell growth in the scar, along with the absence of ECM-secreted factors that would direct neuronal outgrowth, result in very poor regeneration potential and permanent neural dysfunction.
Cell therapy using induced pluripotent stem cell-derived neurons is considered a promising approach to regenerate the injured spinal cord, the team further pointed out. “However, the scar formed at the chronic phase is not a permissive microenvironment for cell or biomaterial engraftment or for tissue assembly.” The researchers hypothesized that mimicking embryonic development by applying what they describe as “ … a specific spinal cord motor neuron differentiation protocol in a 3D dynamic microenvironment …” would provide the cells not only differentiation cues, but also signals for appropriate tissue formation with natural hallmarks. “We further hypothesized that assembling a functional neuronal network prior to implantation would increase the chances of functional engraftment.”

In their newly published paper, the investigators described how they had recently developed iPSCs-derived human tissue implants with the potential to perfectly match the immunological and cellular profile of a patient. “In principle, in this approach, a small piece of fatty tissue biopsy is extracted from a patient and the cellular and acellular materials are separated,” they explained. “While the cells are reprogrammed to become iPSCs, the ECM is processed to become a personalized hydrogel.”
“Our technology is based on taking a small biopsy of belly fat tissue from the patient,” Dvir stated. “This tissue, like all tissues in our body, consists of cells together with an ECM (comprising substances like collagens and sugars). After separating the cells from the ECM we used genetic engineering to reprogram the cells, reverting them to a state that resembles embryonic stem cells—namely cells capable of becoming any type of cell in the body. From the ECM we produced a personalized hydrogel, which would evoke no immune response or rejection after implantation. We then encapsulated the stem cells in the hydrogel and in a process that mimics the embryonic development of the spinal cord we turned the cells into 3D implants of neuronal networks containing motor neurons.”
The authors further noted, “Throughout the in vitro cultivation stage, the cells and the hydrogel showed a synergistic effect, mimicking the process of spinal cord formation in the embryo. The processed hydrogel supported the efficient human iPSCs differentiation in 3D by providing the cells with an adequate microenvironment. Subsequently, during the differentiation, cells at different developmental stages continuously remodeled the hydrogel by secreting specific neuronal ECM proteins, providing an inductive microenvironment for cell-cell and cell-matrix interactions.”
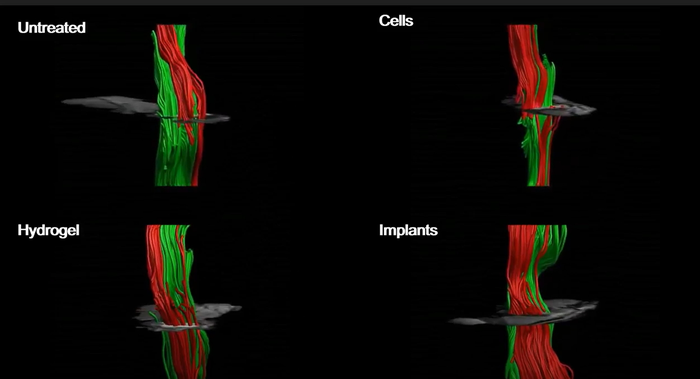
The resulting human spinal cord implants were tested in murine lab models of paralysis. As proof of concept, the researchers first successfully tested the implants in a mouse model of acute injury and paralysis. “The implants enriched the hindered region with biochemical and mechanical cues to attract progenitor cells, supported cell survival and engraftment, reduced inflammation and gliosis at the lesion site, and overall improved the locomotion of the treated animal,” the team wrote.
They also tested the implants in a mouse model of chronic, or longer-term injury and paralysis, equivalent to a year in human terms. “Having demonstrated the ability of the implants to recover the injured spinal cord in the acute phase, we moved on to assess their ability to regenerate the tissue in a more clinically relevant model,” the authors pointed out. “At this stage, the scar is fully developed and spontaneous behavioral recovery has reached its plateau.” The results confirmed that following implantation, 100% of the lab models with acute paralysis, and 80% of those with chronic paralysis, regained their ability to walk.
Dvir further noted, “The model animals underwent a rapid rehabilitation process, at the end of which they could walk quite well … There are millions of people around the world who are paralyzed due to spinal injury, and there is still no effective treatment for their condition. Individuals injured at a very young age are destined to sit in a wheelchair for the rest of their lives, bearing all the social, financial, and health-related costs of paralysis. Our goal is to produce personalized spinal cord implants for every paralyzed person, enabling regeneration of the damaged tissue with no risk of rejection.”

In their published paper, the authors concluded, “Looking forward, after overcoming strict regulation challenges, the reported technology may be relevant for treating paralyzed human patients. In this concept, by taking a small biopsy from the patient, a personalized hydrogel can be produced and patient-specific iPSCs can be generated to obtain personalized SC implants. The ability of the implants to replace the resected scar tissue and rewire the injured SC of humans may represent a novel personalized cell therapy approach.”
Dvir has teamed up with industry partners to establish Matricelf, a company that is applying the approach with the goal of making spinal cord implant treatments commercially available for people with paralysis. “The company’s preclinical program has already been discussed with the FDA,” Dvir said. “Since we are proposing an advanced technology in regenerative medicine, and since at present there is no alternative for paralyzed patients, we have good reason to expect relatively rapid approval of our technology.”news