The FDA has lifted a partial clinical hold imposed in April on Epizyme’s cancer treatment tazemetostat, as well as the clinical hold carried out in July on Sarepta Therapeutics’ Phase I/IIa trial of its Duchenne Muscular Dystrophy (DMD) gene therapy candidate AAVrh74.MHCK7.micro-Dystrophin, the companies said.
The FDA paused enrollment in U.S.-based enrollment of new patients in clinical trials of tazemetostat, Epizyme’s lead candidate, after one patient in a Phase I pediatric study (NCT02601937) developed T-cell lymphoblastic lymphoma (T-LBL).
The patient “had advanced poorly differentiated chordoma” and was the only instance of secondary lymphoma that has been observed across the tazemetostat clinical program, Epizyme said when the partial hold was imposed: “At the time of the safety report, the patient had been on study for approximately 15 months and had achieved a confirmed partial response. This patient has now discontinued tazemetostat and is being treated for T-cell lymphoma.”
Today, Epizyme said it followed up on the T-LBL patient through a formal response to the FDA that included a comprehensive assessment of the risk of secondary malignancies, including T-LBL potentially associated with tazemetostat, taking into account both published literature and the company’s clinical experience to date.
Epizyme said it provided a thorough assessment of efficacy and safety data across all of its trials in hematological malignancies and solid tumors, in both adults and children, and convened a panel of external scientific and medical experts who reviewed and validated the findings.
Reopening Enrollment
In addition, Epizyme said, it is in the process of reopening enrollment in all of its company-sponsored trials in the U.S., including the follicular lymphoma (FL) EZH2 activating mutation cohort of its Phase II non-Hodgkin lymphoma trial.
“The Epizyme team has worked diligently to provide a comprehensive response back to the FDA, and through constructive dialogue, we successfully resolved the partial clinical hold. This allows us to turn our full attention to our key priorities: preparing for our first NDA submission for tazemetostat in epithelioid sarcoma and defining our registration path in FL,” Epizyme president and CEO Robert Bazemore said in a statement.
Tazemetostat is a first-in-class enhancer of zeste homolog 2 (EZH2) inhibitor under study as a monotherapy in ongoing Phase II programs in solid tumors that include epithelioid sarcoma and other INI1-negative tumors, and follicular lymphoma (FL)—as well as in combination studies in diffuse large B-cell lymphoma (DLBCL) and non-small cell lung cancer (NSCLC).
According to Clinicaltrias.gov, tazemetostat is being assessed in seven active studies that are recruiting patients; five other studies that were active but not recruiting patients; and three studies that had been suspended as of their most recent updates.
Suspended were:
- A Phase I National Cancer Institute (NCI) trial (NCT03217253) designed to assess the best dose and side effect of tazemetostat in treating patients with solid tumors or B-cell lymphomas with liver dysfunction that have spread to other places in the body or cannot be removed by surgery. (Suspended as of July 18).
- A Phase II NCI Pediatric MATCH trial (NCT03213665 ) designed to study how well tazemetostat works in treating patients with solid tumors, non-Hodgkin lymphoma, or histiocytic disorders that have spread to other places in the body and have come back or do not respond to treatment and have EZH2, SMARCB1, or SMARCA4 gene mutations.
- A Phase I/II trial (NCT02889523) by Epizyme and The Lymphoma Academic Research Organisation. Phase I is designed to determine the recommended Phase II dose for tazemetostat in patients treated with R-CHOP 21 chemotherapy, while Phase II is designed to determine the safety of tazemetostat in patients treated with eight cycles of R-CHOP 21, as well as complete response rate according to Cheson International Working Group (IWG) 2014: Lugano Classification after 8 cycles of Epi-RCHOP 21.
‘Trace’ Triggers Sarepta Hold
Sarepta’s Phase I/IIa trial (NCT03375164) of AAVrh74.MHCK7.micro-Dystrophin was placed on clinical hold after a “trace” amount of DNA fragment surfaced in a manufacturing lot of research-grade raw material plasmid supplied by an undisclosed third-party manufacturer.
Today, Sarepta said its clinical hold was lifted after joining with its partner in the clinical trial, Nationwide Children’s Hospital, to develop and submit an action plan to the FDA, including an audit of the plasmid supplier and a commitment to use GMP-s plasmid for all future production lots.
“Thanks to the diligent and rapid work of my Sarepta colleagues and Nationwide Children’s Hospital in compiling and submitting a complete response and the expeditious evaluation by the FDA in reviewing the response and removing this clinical hold, we have been able to address the clinical hold in record time and without delay to this profoundly important clinical program,” Sarepta president and CEO Doug Ingram stated.
“Our focus now is on meeting with the [FDA] to take guidance and gain alignment around what we hope to be our registration trial for our micro-dystrophin program and achieving our goal of commencing that trial by year-end 2018,” Ingram added.
The trial is one of two clinical studies of AAVrh74.MHCK7.micro-Dystrophin listed on ClinicalTrials.gov. Nationwide Children’s is also overseeing a Phase I double-blind, randomized controlled study (NCT02710500) assessing the gene therapy as a treatment for dysferlinopathies, with direct intramuscular injection of rAAVrh.74.MHCK7.DYSF.DV gene vector to the extensor digitorum brevis muscle (EDB). The trial was active but not recruiting patients as of the most recent update, posted July 18.
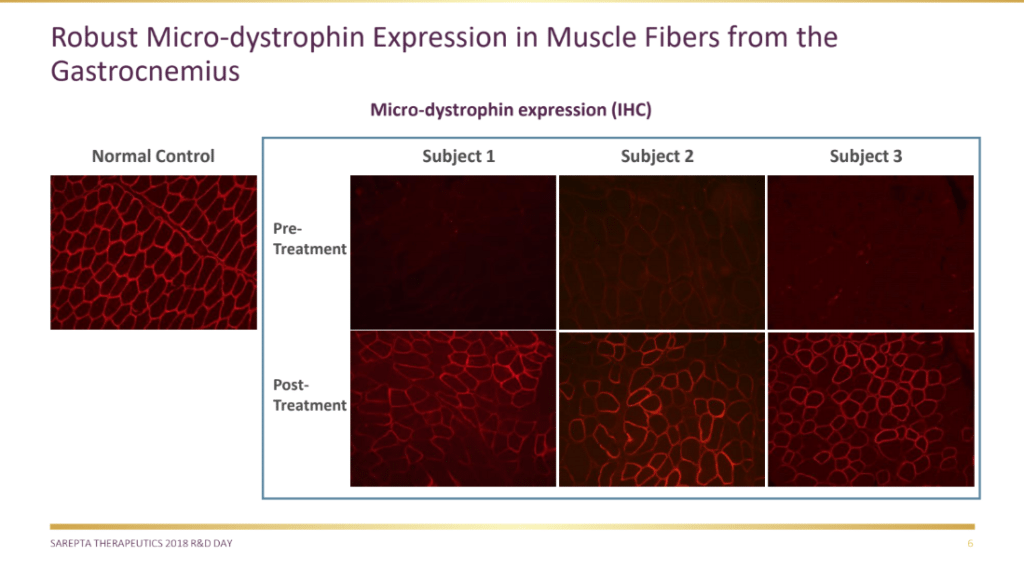
A month earlier on June 19, Sarepta trumpeted positive preliminary clinical data for AAVrh74.MHCK7.micro-Dystrophin in the Phase I/IIa study. Sarepta announced that all three patients showed robust micro-dystrophin expression in muscle measured by all methods, as well as significant decreases in the levels of serum creatine kinase (CK), an enzyme biomarker strongly associated with muscle damage caused by DMD. The mean reduction of CK was over 87% at day 60.