“CRISPR technology has empowered our team to innovate a new, effective, species-specific, self-limiting, safe, and scalable genetic population control technology with remarkable potential to be developed and utilized in a plethora of insect pests and disease vectors,” said Omar Akbari, PhD, an assistant professor in UC San Diego’s division of biological sciences and corresponding author. “In the future, we strongly believe this technology will be safely used in the field to suppress and even eradicate target species locally, thereby revolutionizing how insects are managed and controlled going forward.” The researchers report their developments in a paper titled, “Transforming insect population control with precision-guided sterile males with demonstration in flies.”
Methods for releasing sterile male insects into the wild to control and eradicate pest populations have been used since the 1930s. A strategy based on irradiated males was applied in the U.S. in the 1950s to eliminate the New World Screwworm fly, and radiation-based methods continue to be used today.
CRISPR-based genome editing has “revolutionized” the ability to carry out precise genome editing in just about every organism studied, the researchers noted. Recent developments include homing-based gene drives that propagate genetic changes through subsequent generations, and which have spawned a field known as Active Genetics. While such techniques could feasibly be used to combat disease-carrying insect vectors or control invasive species and crop pests, “ongoing discussions are underway to define the mechanisms of governance to ensure that the technology is ethically, and safely, developed and implemented,” the authors noted. Current gene drive designs also demonstrate drawbacks, including the rapid evolution of resistance, they continued, “and therefore future research is necessary to develop drives that can limit and overcome evolved resistance.”
Given these considerations, Akbari and colleagues set out to develop a “novel, safe and controllable, noninvasive, CRISPR-based genetic technology that could be transferred across species and implemented worldwide in the short term to combat wild populations.”
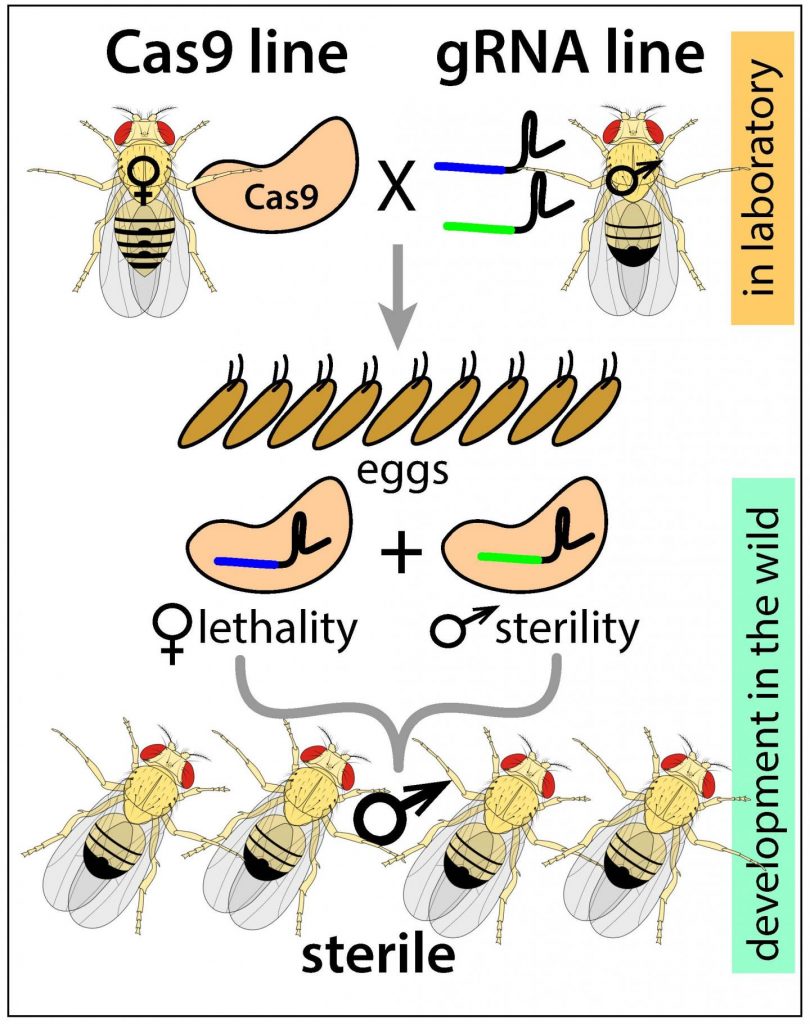
The resulting pgSIT strategy uses CRISPR technology to disrupt key genes that are essential for female viability and for male fertility, and so generate eggs that only give rise to sterile males, and which can then be introduced into the insect population.
Developed initially in Drosophila fruit flies, the technology involves a breeding strategy that requires two homozygous strains, one expressing Cas9 and the other expressing double-guide RNAs (dgRNAs), the authors commented. A single mating between the strains simultaneously knocks out a gene that is required for female viability and a gene that is required for male fertility, so only sterile males will survive in the next generation. “A single mating between these strains mechanistically results in synchronous RNA-guided dominant biallelic knockouts of both target genes throughout development, resulting in non-Mendelian complete penetrance of desired phenotypes in all progeny by converting recessive phenotypes into dominant phenotypes in a single generation,” the team stated. Significantly, pgSIT relies on CRISPR-mediated DNA cleavage and NHEJ-based repair, and not homology-direct repair (HDR). “Therefore, generation of resistance alleles that can curtail CRISPR-mediated gene drives does not limit the efficacy of pgSIT …” they stated.
In their published Nature Communications paper, the researchers report on the development of pgSIT in the fruit fly, and describe initial results demonstrating that the technique to be 100% efficient, generating fit, sterile males that could successfully compete for mates. “Importantly, the technology does not rely on chromosome translocations, chemosterilants, irradiation, antibiotics, or bacterial infections, which can severely compromise the fitness and mating competitiveness of released sterile males,” they stated. The targeted genes are also common to a wide cross-section of insects, so the pgSIT technique could feasibly be applied to many different types of insect pests. Critically, mathematical modeling predicted that pgSIT could far more effectively suppress insect populations than can be achieved using current self-limiting technologies. “Taken together, pgSIT offers to potentially transform our ability to control insect agricultural pests and disease vectors,” the team noted. “A single efficient pgSIT egg production facility could distribute pgSIT eggs to many remote field sites all over the world, where they could simply be hatched, reared, and released, reducing costs of building multiple production facilities.”
The pgSIT approach also doesn’t require manual sex sorting of larvae, and population suppression starts immediately when the eggs hatch, as all the larvae are infertile males, which start to consume resources that would otherwise be available to wildtype, fertile larvae.
“This is a novel twist of a very old technology,” said first author Nikolay Kandul, PhD, an assistant project scientist in UC San Diego’s division of biological sciences. “That novel twist makes it extremely portable from one species to another species to suppress populations of mosquitoes or agricultural pests, for example, those that feed on valuable wine grapes.”
Having demonstrated the “simplicity and consistency” of generating sterile males in fruit flies, the researchers hope to develop the pgSIT technology in Aedes aegypti mosquitoes that transmit dengue fever, Zika, yellow fever, and other diseases. “… these results suggest that pgSIT has greater potential to eliminate local Ae. aegypti populations than currently available suppression technologies,” they concluded.






