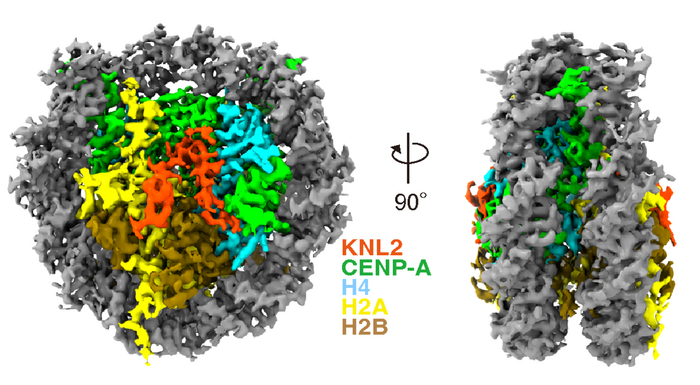
Researchers at Osaka University say they have clarified the structure of the centromeric region in chicken cells using cryogenic electron microscopy (cryo-EM). The team published its study in “The cryo-EM structure of the CENP-A nucleosome in complex with ggKNL2” The EMBO Journal.
The results of their work could prove invaluable in advancing knowledge of cell division and growth, according to the scientists, who add that proteins involved in cell division and the kinetochore are targets for anti-cancer drugs. Therefore, this study might also contribute to the design of novel drugs for diseases such as cancer.
“Centromere protein A (CENP-A) nucleosomes containing the centromere-specific histone H3 variant CENP-A represent an epigenetic mark that specifies centromere position. The Mis18 complex is a licensing factor for new CENP-A deposition via the CENP-A chaperone, Holliday junction recognition protein (HJURP), on the centromere chromatin. Chicken KINETOCHORE NULL2 (KNL2) (ggKNL2), a Mis18 complex component, has a CENP-C-like motif, and our previous study suggested that ggKNL2 directly binds to the CENP-A nucleosome to recruit HJURP/CENP-A to the centromere,” write the investigators.
“However, the molecular basis for CENP-A nucleosome recognition by ggKNL2 has remained unclear. Here, we present the cryo-EM structure of the chicken CENP-A nucleosome in complex with a ggKNL2 fragment containing the CENP-C-like motif. Chicken KNL2 distinguishes between CENP-A and histone H3 in the nucleosome using the CENP-C-like motif and its downstream region. Both the C-terminal tail and the RG-loop of CENP-A are simultaneously recognized as CENP-A characteristics.
“The CENP-A nucleosome–ggKNL2 interaction is thus essential for KNL2 functions. Furthermore, our structural, biochemical, and cell biology data indicate that ggKNL2 changes its binding partner at the centromere during chicken cell cycle progression.”
Cryo-EM technology proves critical
Cryo-EM freezes samples quickly to preserve and stabilize them, and then images them using collisions with electrons to reveal their structure. A complex of proteins (kinetochore) forms at the centromeric region, and this is essential for cells to divide correctly. The researchers were able to clarify a structural change to the kinetochore at the atomic level using cryo-EM analysis.
The research team showed that during mitosis, the CENP-C protein binds CENP-A and acts as a scaffold for other kinetochore proteins. However, during interphase, a different protein (KNL2) binds to centromeres instead.
“KNL2 contains a CENP-C-like motif and is a component of the Mis18 complex, a licensing factor for new CENP-A deposition,” explains lead authors of the study Honghui Jiang, a doctoral student, and Mariko Ariyoshi, Pharm D.
The team further revealed that this interaction between KNL2 and the centromere is required for new deposition of CENP-A during interphase, which in turn helps maintain the correct location of the centromere.
“We also showed that CENP-C is phosphorylated during mitosis, and phosphorylated CENP-C excludes KNL2 from the KNL2–CENP-A complex,” notes senior author Tatsuo Fukagawa, PhD, who suggests that KNL2 binds to CENP-A through interphase, maintaining the location of the centromere until a phosphate molecule becomes bound to CENP-C as the cells reach mitosis. Then, CENP-C preferentially binds to CENP-A, allowing the formation of the kinetochore for cell division.