Some of the DNA in cancer cells may be found in circular structures, rings of extrachromosomal DNA, or ecDNA, that may unloose all manner of mayhem. In fact, ecDNA is a sort of DNA-ring circus. The performers are oncogenes, the genetic equivalent of reckless acrobats, rampant beasts, and evil clowns. They are given to free expression.
ecDNA rings happen to be abundant in tumor cells, where they promote malignant behavior, report scientists based at the University of California, San Diego (UCSD).
The scientists, led by Paul S. Mischel, MD, professor of pathology at UCSD, have been studying ecDNA for years. Back in 2014, they discovered that ecDNA plays a central role in the drug resistance of certain brain tumors by enabling tumors to rapidly change the amount of oncogenes they contain—and thereby determine whether a cell transforms into a tumor cell. The finding surprised cancer biologists who had long focused more on which genes promote cancer rather than where those genes are located.
The Mischel group’s latest findings dramatically underscore how cancer cells don’t play by the same biological rules as eukaryotic cells. Rather than passing DNA to subsequent generations by dividing into genetically identical daughter cells—a process called mitosis, involving paired chromosomes that divide and used by all eukaryotes—cancer cells propagate somewhat like bacterial cells. That is, the cancer cells parcel out ecDNA to daughter cells in a seemingly random way, providing a mechanism by which certain daughter cells could receive multiple cancerous copies within one cell division. It is a distinctly different process of inheritance which allows for more rapid evolution and genetic change.
As if that weren’t bad enough, ecDNA has another trick up its sleeve. According to Mischel and colleagues, ecDNA is more loosely packed and hence more accessible than chromosomal DNA. With ecDNA, oncogenes are more likely to be expressed at high levels.
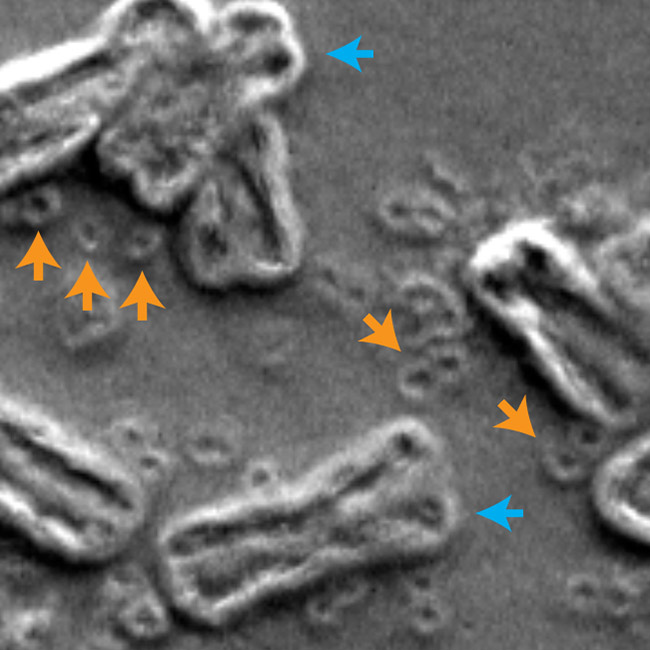
The new findings appeared November 20 in Nature, in an article titled, “Circular ecDNA promotes accessible chromatin and high oncogene expression.” The paper describes how Mischel and colleagues demonstrated the structure of circular DNA by integrating ultrastructural imaging, long-range optical mapping, and computational analysis of whole-genome sequencing. The paper also presents evidence that oncogenes encoded on ecDNA are among the most highly expressed genes in the transcriptome of the tumors.
“Quantitative assessment of the chromatin state reveals that although ecDNA is packaged into chromatin with intact domain structure, it lacks higher-order compaction that is typical of chromosomes and displays significantly enhanced chromatin accessibility,” the article’s authors wrote. “Furthermore, ecDNA is shown to have a significantly greater number of ultra-long-range interactions with active chromatin, which provides insight into how the structure of circular ecDNA affects oncogene function, and connects ecDNA biology with modern cancer genomics and epigenetics.”
The authors suggest that ecDNA, because of its increased DNA copy numbers and in association with enhanced chromatin accessibility, promotes massively increased transcription of the oncogenes. More generally, ecDNA contributes to cancer pathogenesis by altering the shape of its chromatin.
“This is a paradigm shift,” said Mischel. “The shape of cancer ecDNA is different than normal DNA, and that has really important implications, both for our understanding of cancer biology and clinical impact.”
Human DNA typically forms long, twisting double helices of genetic material: roughly three billion base pairs organized into 23 pairs of chromosomes miraculously squeezed into every cell nucleus, each averaging just six micrometers in diameter. (Laid out end-to-end, all of the DNA in a single cell nucleus would extend roughly six feet and all of the DNA in one person’s body would span roughly twice the diameter of the solar system, more than seven billion miles.)
In humans and other eukaryote organisms, normal DNA is packed into cell nuclei by tightly wrapping it around closely bunched clusters of protein complexes called histone octamers. In order to access and read DNA’s genetic instructions, cells rely on enzymes and complicated machinery to cut and move bits and pieces, making only portions accessible at a time, not unlike reading a partially opened scroll.
Although ecDNA is wound around protein cores, it is highly accessible, allowing for more access points and places where genetic information can be quickly transcribed and expressed. This feature enables tumor cells to generate massive amounts of growth-promoting oncogenes—and evolve more quickly and respond more forcefully to their changing environment and potential threats.
“By showing that ecDNA is circular, then elucidating its epigenetic organization, we demonstrate something very powerful,” stated Mischel. “This unique shape in human cancer cells is quite unlike normal human DNA. It really shines a new light onto the 3D organization of the screwed-up cancer genome and epigenome, which now provides a mechanistic basis for understanding why certain tumor cells are so aggressive.”

